Composition of intraperitoneally implanted electrospun conduits modulates cellular elastic matrix generation
- PMID: 24016842
- PMCID: PMC4024661
- DOI: 10.1016/j.actbio.2013.08.042
Composition of intraperitoneally implanted electrospun conduits modulates cellular elastic matrix generation
Abstract
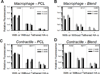
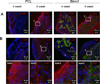
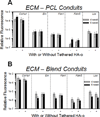
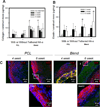
Improving elastic matrix generation is critical to developing functional tissue engineered vascular grafts. Therefore, this study pursued a strategy to grow autologous tissue in vivo by recruiting potentially more elastogenic cells to conduits implanted within the peritoneal cavity. The goal was to determine the impacts of electrospun conduit composition and hyaluronan oligomer (HA-o) modification on the recruitment of peritoneal cells, and their phenotype and ability to synthesize elastic matrix. These responses were assessed as a function of conduit intra-peritoneal implantation time. This study showed that the blending of collagen with poly(ε-caprolactone) (PCL) promotes a faster wound healing response, as assessed by trends in expression of macrophage and smooth muscle cell (SMC) contractile markers and in matrix deposition, compared to the more chronic response for PCL alone. This result, along with the increase in elastic matrix production, demonstrates the benefits of incorporating as little as 25% w/w collagen into the conduit. In addition, PCR analysis demonstrated the challenges in differentiating between a myofibroblast and an SMC using traditional phenotypic markers. Finally, the impact of the tethered HA-o is limited within the inflammatory environment, unlike the significant response found previously in vitro. In conclusion, these results demonstrate the importance of both careful control of implanted scaffold composition and the development of appropriate delivery methods for HA-o.
Keywords: Collagen; Elastin; Electrospinning; Peritoneal cavity; Vascular grafts.
Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Temporal changes in peritoneal cell phenotype and neoelastic matrix induction with hyaluronan oligomers and TGF-β1 after implantation of engineered conduits.J Tissue Eng Regen Med. 2018 Jun;12(6):1420-1431. doi: 10.1002/term.2674. Epub 2018 May 18. J Tissue Eng Regen Med. 2018. PMID: 29701914
-
Aligned electrospun scaffolds and elastogenic factors for vascular cell-mediated elastic matrix assembly.J Tissue Eng Regen Med. 2012 Oct;6(9):673-86. doi: 10.1002/term.470. Epub 2011 Sep 23. J Tissue Eng Regen Med. 2012. PMID: 21953981 Free PMC article.
-
Impact of electrospun conduit fiber diameter and enclosing pouch pore size on vascular constructs grown within rat peritoneal cavities.Tissue Eng Part A. 2013 Apr;19(7-8):809-23. doi: 10.1089/ten.TEA.2012.0309. Epub 2012 Nov 30. Tissue Eng Part A. 2013. PMID: 23075322 Free PMC article.
-
Collagen incorporation within electrospun conduits reduces lipid oxidation and impacts conduit mechanics.Biomed Mater. 2016 Apr 21;11(2):025019. doi: 10.1088/1748-6041/11/2/025019. Biomed Mater. 2016. PMID: 27099237
-
Tissue engineering and regenerative strategies to replicate biocomplexity of vascular elastic matrix assembly.Tissue Eng Part B Rev. 2012 Jun;18(3):203-17. doi: 10.1089/ten.TEB.2011.0521. Epub 2012 Mar 2. Tissue Eng Part B Rev. 2012. PMID: 22224468 Free PMC article. Review.
Cited by
-
Magnetically Responsive Bone Marrow Mesenchymal Stem Cell-Derived Smooth Muscle Cells Maintain Their Benefits to Augmenting Elastic Matrix Neoassembly.Tissue Eng Part C Methods. 2016 Apr;22(4):301-11. doi: 10.1089/ten.TEC.2015.0349. Epub 2016 Mar 18. Tissue Eng Part C Methods. 2016. PMID: 26830683 Free PMC article.
-
Novel electrospun poly(ε-caprolactone)/type I collagen nanofiber conduits for repair of peripheral nerve injury.Neural Regen Res. 2019 Sep;14(9):1617-1625. doi: 10.4103/1673-5374.255997. Neural Regen Res. 2019. PMID: 31089062 Free PMC article.
References
-
- Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–362. - PubMed
-
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–2828. - PubMed
-
- Fornieri C, Quaglino D, Jr, Mori G. Role of the extracellular matrix in age-related modifications of the rat aorta. Ultrastructural, morphometric, and enzymatic evaluations. Arterioscler Thromb. 1992;12:1008–1016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

