Cardiomyocyte-secreted acetylcholine is required for maintenance of homeostasis in the heart
- PMID: 24018063
- PMCID: PMC3834786
- DOI: 10.1096/fj.13-238279
Cardiomyocyte-secreted acetylcholine is required for maintenance of homeostasis in the heart
Abstract
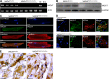
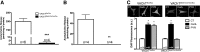
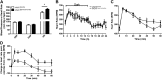
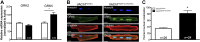
Heart activity and long-term function are regulated by the sympathetic and parasympathetic branches of the nervous system. Parasympathetic neurons have received increased attention recently because acetylcholine (ACh) has been shown to play protective roles in heart disease. However, parasympathetic innervation is sparse in the heart, raising the question of how cholinergic signaling regulates cardiomyocytes. We hypothesized that non-neuronal secretion of ACh from cardiomyocytes plays a role in cholinergic regulation of cardiac activity. To test this possibility, we eliminated secretion of ACh exclusively from cardiomyocytes by targeting the vesicular acetylcholine transporter (VAChT). We find that lack of cardiomyocyte-secreted ACh disturbs the regulation of cardiac activity and causes cardiomyocyte remodeling. Mutant mice present normal hemodynamic parameters under nonstressful conditions; however, following exercise, their heart rate response is increased. Moreover, hearts from mutant mice present increased oxidative stress, altered calcium signaling, remodeling, and hypertrophy. Hence, without cardiomyocyte-derived ACh secretion, hearts from mutant mice show signs of imbalanced autonomic activity consistent with decreased cholinergic drive. These unexpected results suggest that cardiomyocyte-derived ACh is required for maintenance of cardiac homeostasis and regulates critical signaling pathways necessary to maintain normal heart activity. We propose that this non-neuronal source of ACh boosts parasympathetic cholinergic signaling to counterbalance sympathetic activity regulating multiple aspects of heart physiology.
Keywords: VAChT; autonomic function; cardiac hypertrophy; cardiac remodeling; choline acetyltransferase; parasympathetic activity.
Figures



Comment in
-
Letters to the editor: Cardiomyocyte-secreted acetylcholine.FASEB J. 2014 Jan;28(1):1-2. doi: 10.1096/fj.14-0101LTE. FASEB J. 2014. PMID: 24385568 No abstract available.
-
Response.FASEB J. 2014 Jan;28(1):2-3. doi: 10.1096/fj.14-0102LTE. FASEB J. 2014. PMID: 24385569 No abstract available.
References
-
- Levy M. N. (1997) Neural control of cardiac function. Baillieres Clin. Neurol. 6, 227–244 - PubMed
-
- Kanazawa H., Ieda M., Kimura K., Arai T., Kawaguchi-Manabe H., Matsuhashi T., Endo J., Sano M., Kawakami T., Kimura T., Monkawa T., Hayashi M., Iwanami A., Okano H., Okada Y., Ishibashi-Ueda H., Ogawa S., Fukuda K. (2010) Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J. Clin. Invest. 120, 408–421 - PMC - PubMed
-
- Lara A., Damasceno D. D., Pires R., Gros R., Gomes E. R., Gavioli M., Lima R. F., Guimaraes D., Lima P., Bueno C. R., Jr., Vasconcelos A., Roman-Campos D., Menezes C. A., Sirvente R. A., Salemi V. M., Mady C., Caron M. G., Ferreira A. J., Brum P. C., Resende R. R., Cruz J. S., Gomez M. V., Prado V. F., de Almeida A. P., Prado M. A., Guatimosim S. (2010) Dysautonomia due to reduced cholinergic neurotransmission causes cardiac remodeling and heart failure. Mol. Cell. Biol. 30, 1746–1756 - PMC - PubMed
-
- Hoover D. B., Ganote C. E., Ferguson S. M., Blakely R. D., Parsons R. L. (2004) Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc. Res. 62, 112–121 - PubMed
-
- Kawano H., Okada R., Yano K. (2003) Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 18, 32–39 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

