Driving vascular endothelial cell fate of human multipotent Isl1+ heart progenitors with VEGF modified mRNA
- PMID: 24018375
- PMCID: PMC3790234
- DOI: 10.1038/cr.2013.112
Driving vascular endothelial cell fate of human multipotent Isl1+ heart progenitors with VEGF modified mRNA
Abstract
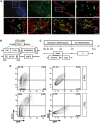
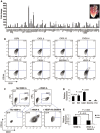
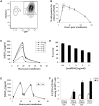
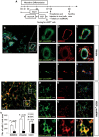
Distinct families of multipotent heart progenitors play a central role in the generation of diverse cardiac, smooth muscle and endothelial cell lineages during mammalian cardiogenesis. The identification of precise paracrine signals that drive the cell-fate decision of these multipotent progenitors, and the development of novel approaches to deliver these signals in vivo, are critical steps towards unlocking their regenerative therapeutic potential. Herein, we have identified a family of human cardiac endothelial intermediates located in outflow tract of the early human fetal hearts (OFT-ECs), characterized by coexpression of Isl1 and CD144/vWF. By comparing angiocrine factors expressed by the human OFT-ECs and non-cardiac ECs, vascular endothelial growth factor (VEGF)-A was identified as the most abundantly expressed factor, and clonal assays documented its ability to drive endothelial specification of human embryonic stem cell (ESC)-derived Isl1+ progenitors in a VEGF receptor-dependent manner. Human Isl1-ECs (endothelial cells differentiated from hESC-derived ISL1+ progenitors) resemble OFT-ECs in terms of expression of the cardiac endothelial progenitor- and endocardial cell-specific genes, confirming their organ specificity. To determine whether VEGF-A might serve as an in vivo cell-fate switch for human ESC-derived Isl1-ECs, we established a novel approach using chemically modified mRNA as a platform for transient, yet highly efficient expression of paracrine factors in cardiovascular progenitors. Overexpression of VEGF-A promotes not only the endothelial specification but also engraftment, proliferation and survival (reduced apoptosis) of the human Isl1+ progenitors in vivo. The large-scale derivation of cardiac-specific human Isl1-ECs from human pluripotent stem cells, coupled with the ability to drive endothelial specification, engraftment, and survival following transplantation, suggest a novel strategy for vascular regeneration in the heart.
Figures

Similar articles
-
VEGF regulates relative allocation of Isl1+ cardiac progenitors to myocardial and endocardial lineages.Mech Dev. 2016 Nov;142:40-49. doi: 10.1016/j.mod.2016.10.004. Epub 2016 Oct 26. Mech Dev. 2016. PMID: 27794491
-
Prospective Isolation of ISL1+ Cardiac Progenitors from Human ESCs for Myocardial Infarction Therapy.Stem Cell Reports. 2018 Mar 13;10(3):848-859. doi: 10.1016/j.stemcr.2018.01.037. Epub 2018 Mar 1. Stem Cell Reports. 2018. PMID: 29503094 Free PMC article.
-
Origin of non-cardiac endothelial cells from an Isl1+ lineage.FEBS Lett. 2012 Jun 21;586(13):1790-4. doi: 10.1016/j.febslet.2012.05.014. Epub 2012 May 18. FEBS Lett. 2012. PMID: 22613570
-
Biology of Isl1+ cardiac progenitor cells in development and disease.Cell Mol Life Sci. 2007 Mar;64(6):674-82. doi: 10.1007/s00018-007-6520-5. Cell Mol Life Sci. 2007. PMID: 17380308 Free PMC article. Review.
-
Islet1-expressing cardiac progenitor cells: a comparison across species.Dev Genes Evol. 2013 Mar;223(1-2):117-29. doi: 10.1007/s00427-012-0400-1. Epub 2012 Apr 24. Dev Genes Evol. 2013. PMID: 22526874 Free PMC article. Review.
Cited by
-
New vessel formation in the context of cardiomyocyte regeneration--the role and importance of an adequate perfusing vasculature.Stem Cell Res. 2014 Nov;13(3 Pt B):666-82. doi: 10.1016/j.scr.2014.04.009. Epub 2014 Apr 29. Stem Cell Res. 2014. PMID: 24841067 Free PMC article. Review.
-
Single-cell RNA sequencing of the carotid artery and femoral artery of rats exposed to hindlimb unloading.Cell Mol Life Sci. 2025 Jan 21;82(1):50. doi: 10.1007/s00018-024-05572-x. Cell Mol Life Sci. 2025. PMID: 39833543 Free PMC article.
-
Developmental origin and lineage plasticity of endogenous cardiac stem cells.Development. 2016 Apr 15;143(8):1242-58. doi: 10.1242/dev.111591. Development. 2016. PMID: 27095490 Free PMC article. Review.
-
Biocompatible, Purified VEGF-A mRNA Improves Cardiac Function after Intracardiac Injection 1 Week Post-myocardial Infarction in Swine.Mol Ther Methods Clin Dev. 2018 Apr 10;9:330-346. doi: 10.1016/j.omtm.2018.04.003. eCollection 2018 Jun 15. Mol Ther Methods Clin Dev. 2018. PMID: 30038937 Free PMC article.
-
Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats.Sci Rep. 2018 Aug 22;8(1):12620. doi: 10.1038/s41598-018-30386-w. Sci Rep. 2018. PMID: 30135489 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

