Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury
- PMID: 24018561
- PMCID: PMC3784534
- DOI: 10.1172/JCI67867
Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury
Abstract
Ferritin plays a central role in iron metabolism and is made of 24 subunits of 2 types: heavy chain and light chain. The ferritin heavy chain (FtH) has ferroxidase activity that is required for iron incorporation and limiting toxicity. The purpose of this study was to investigate the role of FtH in acute kidney injury (AKI) and renal iron handling by using proximal tubule-specific FtH-knockout mice (FtH(PT-/-) mice). FtH(PT-/-) mice had significant mortality, worse structural and functional renal injury, and increased levels of apoptosis in rhabdomyolysis and cisplatin-induced AKI, despite significantly higher expression of heme oxygenase-1, an antioxidant and cytoprotective enzyme. While expression of divalent metal transporter-1 was unaffected, expression of ferroportin (FPN) was significantly lower under both basal and rhabdomyolysis-induced AKI in FtH(PT-/-) mice. Apical localization of FPN was disrupted after AKI to a diffuse cytosolic and basolateral pattern. FtH, regardless of iron content and ferroxidase activity, induced FPN. Interestingly, urinary levels of the iron acceptor proteins neutrophil gelatinase-associated lipocalin, hemopexin, and transferrin were increased in FtH(PT-/-) mice after AKI. These results underscore the protective role of FtH and reveal the critical role of proximal tubule FtH in iron trafficking in AKI.
Figures
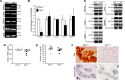

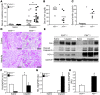



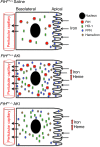
Comment in
-
Acute kidney injury: novel protective role of H-Ferritin.Nat Rev Nephrol. 2013 Nov;9(11):625. doi: 10.1038/nrneph.2013.193. Epub 2013 Oct 1. Nat Rev Nephrol. 2013. PMID: 24080797 No abstract available.
References
-
- Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988;255(3 pt 2):F438–F443. - PubMed
-
- Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988;255(3 pt 2):F539–F544. - PubMed
-
- Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70(3):432–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

