Using Exenatide Twice Daily or Insulin in Clinical Practice: Results from CHOICE
- PMID: 24018835
- PMCID: PMC3889314
- DOI: 10.1007/s13300-013-0037-8
Using Exenatide Twice Daily or Insulin in Clinical Practice: Results from CHOICE
Abstract
Introduction: CHOICE (CHanges to treatment and Outcomes in patients with type 2 diabetes initiating InjeCtablE therapy; NCT00635492) assessed, as its primary objective, the time to a 'significant treatment change' (defined within this paper) after patients with type 2 diabetes mellitus initiated their first injectable, glucose-lowering therapy [exenatide twice daily (BID) or insulin] in clinical practice in six European countries and evaluated outcomes during the study.
Methods: CHOICE was a 24-month, prospective, noninterventional observational study. Patients were invited to participate in CHOICE only after their treating physician had made the clinical decision to initiate first injectable therapy with either exenatide BID or insulin. Clinical data were collected at initiation of first injectable therapy and after approximately 3, 6, 12, 18, and 24 months.
Results: A total of 2,515 patients were recruited; 1,114 patients in the exenatide BID cohort and 1,274 patients in the insulin cohort were eligible for the 24-month analysis. During the study, 42.2% and 36.0% of patients from each cohort, respectively, had a significant treatment change. By 24 months, improved mean glycated hemoglobin (p < 0.001 for both cohorts) and reduced severity of several cardiovascular risk factors were observed in both cohorts; additionally, mean weight was reduced in the exenatide BID cohort (p < 0.001) and increased in the insulin cohort (p < 0.001). Hypoglycemia was reported by 18.4% of the exenatide BID cohort and 36.8% of the insulin cohort; 25.9% of the exenatide BID cohort and 10.0% of the insulin cohort had met the secondary endpoint of glycated hemoglobin <7.0%, no weight gain, and no hypoglycemia.
Conclusion: CHOICE provided data on exenatide BID and insulin usage patterns and 24-month outcomes in clinical practice. On average, improved glycemic control and reduced severity of cardiovascular risk factors were observed in both cohorts, and those in the exenatide BID cohort also had mean weight loss.
Figures

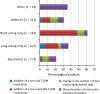

Similar articles
-
Patient-reported outcomes among patients using exenatide twice daily or insulin in clinical practice in six European countries: the CHOICE prospective observational study.Health Qual Life Outcomes. 2013 Dec 26;11:217. doi: 10.1186/1477-7525-11-217. Health Qual Life Outcomes. 2013. PMID: 24369764 Free PMC article.
-
Treatment outcomes after initiation of exenatide twice daily or insulin in clinical practice: 12-month results from CHOICE in six European countries.Diabetes Metab Syndr Obes. 2013 Apr 26;6:171-85. doi: 10.2147/DMSO.S41827. Print 2013. Diabetes Metab Syndr Obes. 2013. PMID: 23667315 Free PMC article.
-
Resource use and costs of exenatide bid or insulin in clinical practice: the European CHOICE study.Clinicoecon Outcomes Res. 2013 Jul 11;5:355-67. doi: 10.2147/CEOR.S44060. Print 2013. Clinicoecon Outcomes Res. 2013. PMID: 23874113 Free PMC article.
-
GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art.Mol Metab. 2021 Apr;46:101102. doi: 10.1016/j.molmet.2020.101102. Epub 2020 Oct 14. Mol Metab. 2021. PMID: 33068776 Free PMC article. Review.
-
[Twice-daily and weekly exenatide: clinical profile of two pioneer formulations in incretin therapy].Med Clin (Barc). 2014 Sep;143 Suppl 2:23-7. doi: 10.1016/S0025-7753(14)70105-8. Epub 2014 Oct 15. Med Clin (Barc). 2014. PMID: 25437462 Review. Spanish.
Cited by
-
Patient-reported outcomes among patients using exenatide twice daily or insulin in clinical practice in six European countries: the CHOICE prospective observational study.Health Qual Life Outcomes. 2013 Dec 26;11:217. doi: 10.1186/1477-7525-11-217. Health Qual Life Outcomes. 2013. PMID: 24369764 Free PMC article.
-
Effects of exenatide in a morbidly obese patient with type 2 diabetes.Diabetes Ther. 2014 Jun;5(1):323-32. doi: 10.1007/s13300-014-0050-6. Epub 2014 Jan 18. Diabetes Ther. 2014. PMID: 24442463 Free PMC article.
-
Exenatide twice daily: a review of its use in the management of patients with type 2 diabetes mellitus.Drugs. 2014 Mar;74(3):325-51. doi: 10.1007/s40265-013-0172-6. Drugs. 2014. PMID: 24435322 Review.
-
Discrete Choice Experiment Attribute Selection Using a Multinational Interview Study: Treatment Features Important to Patients with Type 2 Diabetes Mellitus.Patient. 2017 Aug;10(4):475-487. doi: 10.1007/s40271-017-0225-0. Patient. 2017. PMID: 28315192
-
In vivo degradation forms, anti-degradation strategies, and clinical applications of therapeutic peptides in non-infectious chronic diseases.Eur J Pharmacol. 2022 Oct 15;932:175192. doi: 10.1016/j.ejphar.2022.175192. Epub 2022 Aug 16. Eur J Pharmacol. 2022. PMID: 35981605 Free PMC article. Review.
References
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. - DOI - PubMed
-
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29:2333–2348. doi: 10.1016/j.clinthera.2007.11.006. - DOI - PubMed
-
- Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–267. doi: 10.1007/s00125-006-0510-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

