Identification of BZR1-interacting proteins as potential components of the brassinosteroid signaling pathway in Arabidopsis through tandem affinity purification
- PMID: 24019147
- PMCID: PMC3861714
- DOI: 10.1074/mcp.M113.029256
Identification of BZR1-interacting proteins as potential components of the brassinosteroid signaling pathway in Arabidopsis through tandem affinity purification
Abstract
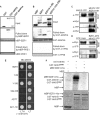
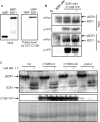
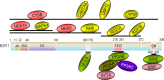
Brassinosteroids (BRs) are essential phytohormones for plant growth and development. BRs are perceived by the cell surface receptor kinase BRI1, and downstream signal transduction through multiple components leads to activation of the transcription factors BZR1 and BZR2/BES1. BZR1 activity is highly controlled by BR through reversible phosphorylation, protein degradation, and nucleocytoplasmic shuttling. To further understand the molecular function of BZR1, we performed tandem affinity purification of the BZR1 complex and identified BZR1-associated proteins using mass spectrometry. These BZR1-associated proteins included several known BR signaling components, such as BIN2, BSK1, 14-3-3λ, and PP2A, as well as a large number of proteins with previously unknown functions in BR signal transduction, including the kinases MKK5 and MAPK4, histone deacetylase 19, cysteine proteinase inhibitor 6, a DEAD-box RNA helicase, cysteine endopeptidases RD21A and RD21B, calmodulin-binding transcription activator 5, ubiquitin protease 12, cyclophilin 59, and phospholipid-binding protein synaptotagmin A. Their interactions with BZR1 were confirmed by in vivo and in vitro assays. Furthermore, MKK5 was found to phosphorylate BZR1 in vitro. This study demonstrates an effective method for purifying proteins associated with low-abundance transcription factors, and identifies new BZR1-interacting proteins with potentially important roles in BR response.
Figures



References
-
- Vert G., Nemhauser J. L., Geldner N., Hong F., Chory J. (2005) Molecular mechanisms of steroid hormone signaling in plants. Annu. Rev. Cell Dev. Biol. 21, 177–201 - PubMed
-
- Wang Z. Y., Bai M. Y., Oh E., Zhu J. Y. (2012) Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 46, 701–724 - PubMed
-
- Li J., Chory J. (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 90, 929–938 - PubMed
-
- Li J., Wen J., Lease K. A., Doke J. T., Tax F. E., Walker J. C. (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 110, 213–222 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

