CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner
- PMID: 24019201
- PMCID: PMC3947164
- DOI: 10.1002/eji.201343509
CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner
Abstract
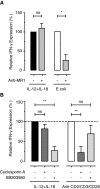
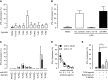
CD161(++) CD8(+) T cells represent a novel subset that is dominated in adult peripheral blood by mucosal-associated invariant T (MAIT) cells, as defined by the expression of a variable-α chain 7.2 (Vα7.2)-Jα33 TCR, and IL-18Rα. Stimulation with IL-18+IL-12 is known to induce IFN-γ by both NK cells and, to a more limited extent, T cells. Here, we show the CD161(++) CD8(+) T-cell population is the primary T-cell population triggered by this mechanism. Both CD161(++) Vα7.2(+) and CD161(++) Vα7.2(-) T-cell subsets responded to IL-12+IL-18 stimulation, demonstrating this response was not restricted to the MAIT cells, but to the CD161(++) phenotype. Bacteria and TLR agonists also indirectly triggered IFN-γ expression via IL-12 and IL-18. These data show that CD161(++) T cells are the predominant T-cell population that responds directly to IL-12+IL-18 stimulation. Furthermore, our findings broaden the potential role of MAIT cells beyond bacterial responsiveness to potentially include viral infections and other inflammatory stimuli.
Keywords: CD161++ T cells; IL-12; IL-18; MAIT cells; T cells.
© 2013 The Authors. European Journal of Immunology published by Wiley-VCH Verlag GmbH & Co. KGaA Weinheim.
Figures



References
-
- Treiner E. Lantz O. CD1d- and MR1-restricted invariant T cells: of mice and men. Curr. Opin. Immunol. 2006;18:519–526. - PubMed
-
- Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, la Salle de H, Bendelac A, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J. Exp. Med. 1999;189:1907–1921. - PMC - PubMed
-
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. - PubMed
-
- Huang S, Gilfillan S, Cella M, Miley MJ, Lantz O, Lybarger L, Fremont DH, et al. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J. Biol. Chem. 2005;280:21183–21193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

