Bio-inspired synthesis yields a tricyclic indoline that selectively resensitizes methicillin-resistant Staphylococcus aureus (MRSA) to β-lactam antibiotics
- PMID: 24019472
- PMCID: PMC3785754
- DOI: 10.1073/pnas.1310459110
Bio-inspired synthesis yields a tricyclic indoline that selectively resensitizes methicillin-resistant Staphylococcus aureus (MRSA) to β-lactam antibiotics
Abstract
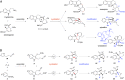
The continuous emergence of resistant bacteria has become a major worldwide health threat. The current development of new antibacterials has lagged far behind. To discover reagents to fight against resistant bacteria, we initiated a chemical approach by synthesizing and screening a small molecule library, reminiscent of the polycyclic indole alkaloids. Indole alkaloids are a class of structurally diverse natural products, many of which were isolated from plants that have been used as traditional medicine for millennia. Specifically, we adapted an evolutionarily conserved biosynthetic strategy and developed a concise and unified diversity synthesis pathway. Using this pathway, we synthesized 120 polycyclic indolines that contain 26 distinct skeletons and a wide variety of functional groups. A tricyclic indoline, Of1, was discovered to selectively potentiate the activity of β-lactam antibiotics in multidrug-resistant methicillin-resistant Staphylococcus aureus (MRSA), but not in methicillin-sensitive S. aureus. In addition, we found that Of1 itself does not have antiproliferative activity but can resensitize several MRSA strains to the β-lactam antibiotics that are widely used in the clinic, such as an extended-spectrum β-lactam antibiotic amoxicillin/clavulanic acid and a first-generation cephalosporin cefazolin. These data suggest that Of1 is a unique selective resistance-modifying agent for β-lactam antibiotics, and it may be further developed to fight against resistant bacteria in the clinic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
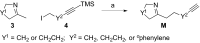

References
-
- Payne DJ. Microbiology. Desperately seeking new antibiotics. Science. 2008;321(5896):1644–1645. - PubMed
-
- McLaughlin JR, Murray CL, Rabinowitz JC. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981;256(21):11283–11291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

