CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo
- PMID: 24019477
- PMCID: PMC3785760
- DOI: 10.1073/pnas.1315006110
CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo
Abstract
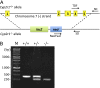
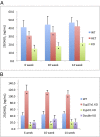
Bioactivation of vitamin D consists of two sequential hydroxylation steps to produce 1α,25-dihydroxyvitamin D3. It is clear that the second or 1α-hydroxylation step is carried out by a single enzyme, 25-hydroxyvitamin D 1α-hydroxylase CYP27B1. However, it is not certain what enzyme or enzymes are responsible for the initial 25-hydroxylation. An excellent case has been made for vitamin D 25-hydroxylase CYP2R1, but this hypothesis has not yet been tested. We have now produced Cyp2r1 (-/-) mice. These mice had greater than 50% reduction in serum 25-hydroxyvitamin D3. Curiously, the 1α,25-dihydroxyvitamin D3 level in the serum remained unchanged. These mice presented no health issues. A double knockout of Cyp2r1 and Cyp27a1 maintained a similar circulating level of 25-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3. Our results support the idea that the CYP2R1 is the major enzyme responsible for 25-hydroxylation of vitamin D, but clearly a second, as-yet unknown, enzyme is another contributor to this important step in vitamin D activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Holick MF. Vitamin D: Evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12(1):4–18. - PubMed
-
- Zhu J, DeLuca HF. Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523(1):30–36. - PubMed
-
- Henry HL. The 25-hydroxyvitamin D 1α-hydroxylase. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. 2nd Ed. Vol 1. San Diego, CA: Elsevier Academic Press; 2005. pp. 69–83.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

