Semisynthetic K+ channels show that the constricted conformation of the selectivity filter is not the C-type inactivated state
- PMID: 24019483
- PMCID: PMC3785774
- DOI: 10.1073/pnas.1308699110
Semisynthetic K+ channels show that the constricted conformation of the selectivity filter is not the C-type inactivated state
Abstract
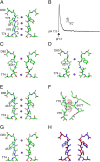
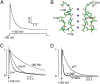
C-type inactivation of K(+) channels plays a key role in modulating cellular excitability. During C-type inactivation, the selectivity filter of a K(+) channel changes conformation from a conductive to a nonconductive state. Crystal structures of the KcsA channel determined at low K(+) or in the open state revealed a constricted conformation of the selectivity filter, which was proposed to represent the C-type inactivated state. However, structural studies on other K(+) channels do not support the constricted conformation as the C-type inactivated state. In this study, we address whether the constricted conformation of the selectivity filter is in fact the C-type inactivated state. The constricted conformation can be blocked by substituting the first conserved glycine in the selectivity filter with the unnatural amino acid d-Alanine. Protein semisynthesis was used to introduce d-Alanine into the selectivity filters of the KcsA channel and the voltage-gated K(+) channel KvAP. For semisynthesis of the KvAP channel, we developed a modular approach in which chemical synthesis is limited to the selectivity filter whereas the rest of the protein is obtained by recombinant means. Using the semisynthetic KcsA and KvAP channels, we show that blocking the constricted conformation of the selectivity filter does not prevent inactivation, which suggests that the constricted conformation is not the C-type inactivated state.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
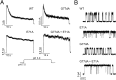


References
-
- MacKinnon R. Nobel Lecture. Potassium channels and the atomic basis of selective ion conduction. Biosci Rep. 2004;24(2):75–100. - PubMed
-
- Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. - PubMed
-
- Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414(6859):43–48. - PubMed
-
- Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: Effects of alterations in the carboxy-terminal region. Neuron. 1991;7(4):547–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

