Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization
- PMID: 24019534
- PMCID: PMC3776352
- DOI: 10.1083/jcb.201301115
Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization
Abstract
Reorganization of the actin cytoskeleton is responsible for dynamic regulation of endothelial cell (EC) barrier function. Circumferential actin bundles (CAB) promote formation of linear adherens junctions (AJs) and tightening of EC junctions, whereas formation of radial stress fibers (RSF) connected to punctate AJs occurs during junction remodeling. The small GTPase Rap1 induces CAB formation to potentiate EC junctions; however, the mechanism underlying Rap1-induced CAB formation remains unknown. Here, we show that myotonic dystrophy kinase-related CDC42-binding kinase (MRCK)-mediated activation of non-muscle myosin II (NM-II) at cell-cell contacts is essential for Rap1-induced CAB formation. Our data suggest that Rap1 induces FGD5-dependent Cdc42 activation at cell-cell junctions to locally activate the NM-II through MRCK, thereby inducing CAB formation. We further reveal that Rap1 suppresses the NM-II activity stimulated by the Rho-ROCK pathway, leading to dissolution of RSF. These findings imply that Rap1 potentiates EC junctions by spatially controlling NM-II activity through activation of the Cdc42-MRCK pathway and suppression of the Rho-ROCK pathway.
Figures
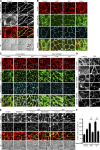
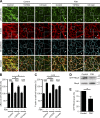


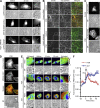
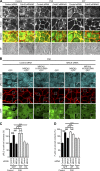


References
-
- Aoki K., Nakamura T., Fujikawa K., Matsuda M. 2005. Local phosphatidylinositol 3,4,5-trisphosphate accumulation recruits Vav2 and Vav3 to activate Rac1/Cdc42 and initiate neurite outgrowth in nerve growth factor-stimulated PC12 cells. Mol. Biol. Cell. 16:2207–2217 10.1091/mbc.E04-10-0904 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

