Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial
- PMID: 24019545
- PMCID: PMC5569677
- DOI: 10.1200/JCO.2012.47.4940
Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial
Abstract
Purpose: Tivozanib is a potent and selective tyrosine kinase inhibitor of vascular endothelial growth factor receptor 1 (VEGFR1), -2, and -3. This phase III trial compared tivozanib with sorafenib as initial targeted therapy in patients with metastatic renal cell carcinoma (RCC).
Patients and methods: Patients with metastatic RCC, with a clear cell component, prior nephrectomy, measurable disease, and 0 or 1 prior therapies for metastatic RCC were randomly assigned to tivozanib or sorafenib. Prior VEGF-targeted therapy and mammalian target of rapamycin inhibitor were not permitted. The primary end point was progression-free survival (PFS) by independent review.
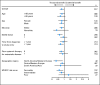
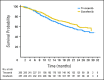
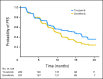
Results: A total of 517 patients were randomly assigned to tivozanib (n = 260) or sorafenib (n = 257). PFS was longer with tivozanib than with sorafenib in the overall population (median, 11.9 v 9.1 months; hazard ratio [HR], 0.797; 95% CI, 0.639 to 0.993; P = .042). One hundred fifty-six patients (61%) who progressed on sorafenib crossed over to receive tivozanib. The final overall survival (OS) analysis showed a trend toward longer survival on the sorafenib arm than on the tivozanib arm (median, 29.3 v 28.8 months; HR, 1.245; 95% CI, 0.954 to 1.624; P = .105). Adverse events (AEs) more common with tivozanib than with sorafenib were hypertension (44% v 34%) and dysphonia (21% v 5%). AEs more common with sorafenib than with tivozanib were hand-foot skin reaction (54% v 14%) and diarrhea (33% v 23%).
Conclusion: Tivozanib demonstrated improved PFS, but not OS, and a differentiated safety profile, compared with sorafenib, as initial targeted therapy for metastatic RCC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Preserving the sanctity of overall survival for drugs approved on the basis of progression-free survival: tivozanib as a case study.J Clin Oncol. 2013 Oct 20;31(30):3746-8. doi: 10.1200/JCO.2013.51.4869. Epub 2013 Sep 9. J Clin Oncol. 2013. PMID: 24019543 No abstract available.
References
-
- Costa LJ, Drabkin HA: Renal cell carcinoma: New developments in molecular biology and potential for targeted therapies Oncologist 12:1404–1415,2007 - PubMed
-
- Escudier B Eisen T Stadler WM, etal: Sorafenib in advanced clear-cell renal-cell carcinoma N Engl J Med 356:125–134,2007 - PubMed
-
- Rini BI Escudier B Tomczak P, etal: Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial Lancet 378:1931–1939,2011 - PubMed
-
- Motzer RJ Hutson TE Tomczak P, etal: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma N Engl J Med 356:115–124,2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

