The effect of different media composition and temperatures on the production of recombinant human growth hormone by CHO cells
- PMID: 24019831
- PMCID: PMC3764673
The effect of different media composition and temperatures on the production of recombinant human growth hormone by CHO cells
Abstract
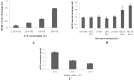
Cell lines derived from mammalian are dominant systems for the production of recombinant therapeutic proteins because of their capacity for correct protein folding, assembly and post-translational modification. In the search of an efficient method for the production of a recombinant protein using animal cell culture, we investigated the effects of different treatment including fetal calf serum concentration, glycerol and culture temperature on a Chinese hamster ovary (CHO) cell line on the production of recombinant human growth hormone (rhGH) and recombinant Chinese hamster ovary (rCHO) viability. The GH production was assessed using ELISA and western blotting methods and cell viability was determined by flow cytometry. The production of recombinant protein increased by 2-fold when stimulatory chemical such as glycerol was added in two stages, first cells were cultured without glycerol for a period of time in order to obtain enough cell density and then glycerol was added to achieve high specific productivity.. Moreover, glycerol addition increased cell viability. Low culture temperature (below 37°C) led to enhanced cellular productivity of the rhGH by 3-fold but decreased cell viability. These findings indicate that quite simple factors such as culture temperature and addition of simple chemicals may lead to the improvement of industrial process for the production of recombinant proteins such as rhGH.
Keywords: Cell viability; Chinese hamster ovary; Culture temperature; FCS; Glycerol; rhGH productivity.
Figures


References
-
- Jayapal KP, Wlaschin KF, Hu W, Yap MGS. Recombinant protein therapeutics from CHO cells-20 years and counting. Chem Eng Prog. 2007;103:140.
-
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat biotechnol. 2004;22:1393–1398. - PubMed
-
- Ryu JH, Kim MS, Lee GM, Choi CY, Kim BS. The enhancement of recombinant protein production by polymer nanospheres in cell suspension culture. Biomaterials. 2005;26:2173–2181. - PubMed
-
- Yoon SK, Hong JK, Choo SH, Song JY, Park HW, Lee GM. Adaptation of Chinese hamster ovary cells to low culture temperature: cell growth and recombinant protein production. J biotechnol. 2006;122:463–472. - PubMed
-
- Fox SR, Patel UA, Yap MGS, Wang DIC. Maximizing interferon production by chinese hamster ovary cells through temperature shift optimization: Experimental and modeling. Biotechnol bioeng. 2004;85:177–184. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
