Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: part - 1
- PMID: 24019854
- PMCID: PMC3767116
- DOI: 10.7150/thno.5922
Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: part - 1
Abstract
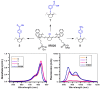
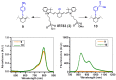
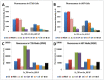
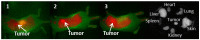
Near-infrared (NIR) organic dyes have become important for many biomedical applications, including in vivo optical imaging. Conjugation of NIR fluorescent dyes to photosensitizing molecules (photosensitizers) holds strong potential for NIR fluorescence image guided photodynamic therapy (PDT) of cancer. Therefore, we were interested in investigating the photophysical properties, in vivo tumor-affinity and fluorescence imaging potential of a series of heterocyclic polymethine dyes, which could then be conjugated to certain PDT agents. For our present study, we selected a series of symmetrical polymethine dyes containing a variety of bis-N-substituted indole or benzindole moieties linked by linear conjugation with and without a fused substituted cyclohexene ring. The N-alkyl side chain at the C-terminal position was functionalized with sulfonic, carboxylic acid, methyl ester or hydroxyl groups. Although, among the parent cyanine dyes investigated, the commercially available, cyanine dye IR783 (3) (bis-indole-N-butylsulfonate)-polymethine dye with a cyclic chloro-cyclohexene moiety showed best fluorescence-imaging ability, based on its spectral properties (λAbs=782 nm, λFl=810 nm, ε = 261,000 M(-1)cm(-1), ΦFl≈0.08) and tumor affinity. In addition to 3, parent dyes IR820 and Cypate (6) were also selected and subjected to further modifications by introducing desired functional groups, which could enable further conjugation of the cyanine dyes to an effective photosensitizer HPPH developed in our laboratory. The synthesis and biological studies (tumor-imaging and PDT) of the resulting bifunctional conjugates are discussed in succeeding paper (Part-2 of this study).
Keywords: Fluorescence Quantum Yields.; Near Infrared Fluorescence Imaging; Near Infrared Fluorophores; Photodynamic therapy; Reactive Oxygen Species.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Core-shell polymeric nanoparticles co-loaded with photosensitizer and organic dye for photodynamic therapy guided by fluorescence imaging in near and short-wave infrared spectral regions.J Nanobiotechnology. 2020 Jan 23;18(1):19. doi: 10.1186/s12951-020-0572-1. J Nanobiotechnology. 2020. PMID: 31973717 Free PMC article.
-
Comparative tumor imaging and PDT Efficacy of HPPH conjugated in the mono- and di-forms to various polymethine cyanine dyes: part - 2.Theranostics. 2013 Aug 21;3(9):703-18. doi: 10.7150/thno.5923. eCollection 2013. Theranostics. 2013. PMID: 24019855 Free PMC article.
-
Measurement of Cyanine Dye Photobleaching in Photosensitizer Cyanine Dye Conjugates Could Help in Optimizing Light Dosimetry for Improved Photodynamic Therapy of Cancer.Molecules. 2018 Jul 24;23(8):1842. doi: 10.3390/molecules23081842. Molecules. 2018. PMID: 30042350 Free PMC article.
-
Development of Polymethine Dyes for NIR-II Fluorescence Imaging and Therapy.Adv Healthc Mater. 2024 Jun;13(16):e2304506. doi: 10.1002/adhm.202304506. Epub 2024 Mar 15. Adv Healthc Mater. 2024. PMID: 38441392 Review.
-
Research progress of near-infrared fluorescence probes based on indole heptamethine cyanine dyes in vivo and in vitro.BMC Chem. 2020 Mar 30;14(1):21. doi: 10.1186/s13065-020-00677-3. eCollection 2020 Dec. BMC Chem. 2020. PMID: 32259133 Free PMC article. Review.
Cited by
-
Theranostics with photodynamic therapy for personalized medicine: to see and to treat.Theranostics. 2023 Oct 9;13(15):5501-5544. doi: 10.7150/thno.87363. eCollection 2023. Theranostics. 2023. PMID: 37908729 Free PMC article. Review.
-
Intraoperative Molecular Imaging in Lung Cancer: The State of the Art and the Future.Mol Ther. 2018 Feb 7;26(2):338-341. doi: 10.1016/j.ymthe.2018.01.013. Epub 2018 Feb 2. Mol Ther. 2018. PMID: 29398484 Free PMC article. No abstract available.
-
Near-Infrared Activated Cyanine Dyes As Agents for Photothermal Therapy and Diagnosis of Tumors.Acta Naturae. 2020 Jul-Sep;12(3):102-113. doi: 10.32607/actanaturae.11028. Acta Naturae. 2020. PMID: 33173600 Free PMC article.
-
Core-shell polymeric nanoparticles co-loaded with photosensitizer and organic dye for photodynamic therapy guided by fluorescence imaging in near and short-wave infrared spectral regions.J Nanobiotechnology. 2020 Jan 23;18(1):19. doi: 10.1186/s12951-020-0572-1. J Nanobiotechnology. 2020. PMID: 31973717 Free PMC article.
-
Comparative tumor imaging and PDT Efficacy of HPPH conjugated in the mono- and di-forms to various polymethine cyanine dyes: part - 2.Theranostics. 2013 Aug 21;3(9):703-18. doi: 10.7150/thno.5923. eCollection 2013. Theranostics. 2013. PMID: 24019855 Free PMC article.
References
-
- Chen X, Conti PS, Moats RA. In vivo Near-Infrared Fluorescence Imaging of Integrin αvβ3 in Brain Tumor Xenografts. Cancer Res. 2004;64:8009–14. - PubMed
-
- Chen Y, Zheng G, Zhang ZH, Blessington D, Zhang M, Li H. et al. Metabolism-enhanced tumor localization by fluorescence imaging: in vivo animal studies. Opt Lett FIELD Full Journal Title:Optics letters. 2003;28:2070–2. - PubMed
-
- Graves EE, Weissleder R, Ntziachristos V. Fluorescence molecular imaging of small animal tumor models. Current Molecular Medicine. 2004;4:419–30. - PubMed
-
- Moon WK, Lin Y, O'Loughlin T, Tang Y, Kim D-E, Weissleder R. et al. Enhanced tumor detection using a folate receptor-targeted near-infrared fluorochrome conjugate. Bioconjugate Chem. 2003;14:539–45. - PubMed
-
- Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nature Biotechnology. 2005;23:313–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

