Beetroot-pigment-derived colorimetric sensor for detection of calcium dipicolinate in bacterial spores
- PMID: 24019934
- PMCID: PMC3760816
- DOI: 10.1371/journal.pone.0073701
Beetroot-pigment-derived colorimetric sensor for detection of calcium dipicolinate in bacterial spores
Abstract
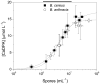
In this proof-of-concept study, we describe the use of the main red beet pigment betanin for the quantification of calcium dipicolinate in bacterial spores, including Bacillus anthracis. In the presence of europium(III) ions, betanin is converted to a water-soluble, non-luminescent orange 1∶1 complex with a stability constant of 1.4 × 10(5) L mol(-1). The addition of calcium dipicolinate, largely found in bacterial spores, changes the color of the aqueous solution of [Eu(Bn)(+)] from orange to magenta. The limit of detection (LOD) of calcium dipicolinate is around 2.0 × 10(-6) mol L(-1) and the LOD determined for both spores, B. cereus and B. anthracis, is (1.1 ± 0.3)× 10(6) spores mL(-1). This simple, green, fast and low cost colorimetric assay was selective for calcium dipicolinate when compared to several analogous compounds. The importance of this work relies on the potential use of betalains, raw natural pigments, as colorimetric sensors for biological applications.
Conflict of interest statement
Figures



References
-
- Vilas-Boas GT, Peruca APS, Arantes OMN (2007) Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Can J Microbiol 53: 673–687. - PubMed
-
- Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. (1999) Anthrax as a biological weapon: Medical and public health management. JAMA, J Am Med Assoc 281: 1735–1745. - PubMed
-
- Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, et al. (2002) Anthrax as a biological weapon, 2002: Updated recommendations for management. JAMA, J Am Med Assoc 287: 2236–2252. - PubMed
-
- Sanderson WT, Stoddard RR, Echt AS, Piacitelli CA, Kim D, et al. (2004) Bacillus anthracis contamination and inhalational anthrax in a mail processing and distribution center. J Appl Microbiol 96: 1048–1056. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

