Basic amino acid mutations in the nuclear localization signal of hibiscus chlorotic ringspot virus p23 inhibit virus long distance movement
- PMID: 24019944
- PMCID: PMC3760818
- DOI: 10.1371/journal.pone.0074000
Basic amino acid mutations in the nuclear localization signal of hibiscus chlorotic ringspot virus p23 inhibit virus long distance movement
Abstract
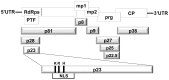
The p23 is a unique protein in the Hibiscus chlorotic ringspot virus which belongs to Family Tombusviridae Genus Carmovirus. Our previous results showed that the p23 is indispensable for host-specific replication and is localized in the nucleus with a novel nuclear localization signal. To investigate additional function(s) of p23, mutations of basic amino acids lysine (K), arginine (R) and histidine (H) that abolish its nuclear localization, were introduced into a biologically active full-length cDNA clone p223 of HCRSV for testing its effects on virus replication and virus movement in vivo. Primer-specific reverse transcription-PCR was conducted to detect gene transcript level of p23 and viral coat protein separately. Virus replication and its coat protein expression were detected by fluorescent in situ hybridization and Western blot, respectively. The effect of p23 was further confirmed by using artificial microRNA inoculation-mediated silencing. Results showed that the two mutants were able to replicate in protoplasts but unable to move from inoculated leaves to newly emerged leaves. Both the p23 and the CP genes of HCRSV were detected in the newly emerged leaves of infected plants but CP was not detected by Western blot and no symptom was observed on those leaves at 19 days post inoculation. This study demonstrates that when p23 is prevented from entering the nucleus, it results in restriction of virus long distance movement which in turn abrogates symptom expression in the newly emerged leaves. We conclude that the p23 protein of HCRSV is required for virus long distance movement.
Conflict of interest statement
Figures


Similar articles
-
The p23 protein of hibiscus chlorotic ringspot virus is indispensable for host-specific replication.J Virol. 2002 Dec;76(23):12312-9. doi: 10.1128/jvi.76.23.12312-12319.2002. J Virol. 2002. PMID: 12414971 Free PMC article.
-
Hibiscus chlorotic ringspot virus coat protein is essential for cell-to-cell and long-distance movement but not for viral RNA replication.PLoS One. 2014 Nov 17;9(11):e113347. doi: 10.1371/journal.pone.0113347. eCollection 2014. PLoS One. 2014. PMID: 25402344 Free PMC article.
-
Hibiscus chlorotic ringspot virus p27 and its isoforms affect symptom expression and potentiate virus movement in kenaf (Hibiscus cannabinus L.).Mol Plant Microbe Interact. 2006 Sep;19(9):948-57. doi: 10.1094/MPMI-19-0948. Mol Plant Microbe Interact. 2006. PMID: 16941899
-
Host-induced avirulence of hibiscus chlorotic ringspot virus mutants correlates with reduced gene-silencing suppression activity.J Gen Virol. 2006 Feb;87(Pt 2):451-459. doi: 10.1099/vir.0.81578-0. J Gen Virol. 2006. PMID: 16432034
-
Identification of a plant viral RNA genome in the nucleus.PLoS One. 2012;7(11):e48736. doi: 10.1371/journal.pone.0048736. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155403 Free PMC article.
Cited by
-
Loss of ZNF32 augments the regeneration of nervous lateral line system through negative regulation of SOX2 transcription.Oncotarget. 2016 Oct 25;7(43):70420-70436. doi: 10.18632/oncotarget.11895. Oncotarget. 2016. PMID: 27626680 Free PMC article.
-
Characterization of Hibiscus Chlorotic Ringspot Virus-Derived vsiRNAs from Infected Hibiscus rosa-sinensis in China.Plant Pathol J. 2024 Oct;40(5):415-424. doi: 10.5423/PPJ.OA.06.2024.0090. Epub 2024 Oct 1. Plant Pathol J. 2024. PMID: 39397297 Free PMC article.
References
-
- You XJ, Kim JW, Stuart GW, Bozarth RF (1995) The nucleotide sequence of Cowpea mottle virus and its assignment to the genus Carmovirus. J Gen Virol 76 2841–2845. - PubMed
-
- Riviere CJ, Rochon DM (1990) Nucleotide sequence and genomic organization of Melon necrotic spot virus. J Gen Virol 71 1887–1896. - PubMed
-
- Berthome R, Kusiak C, Renou JP, Albouy J, Freire MA, et al. (1998) Relationship of the Pelargonium flower break carmovirus (PFBV) coat protein gene with that of other carmoviruses . Arch Virol 143: 1823–1829. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

