SIRT3: A Central Regulator of Mitochondrial Adaptation in Health and Disease
- PMID: 24020003
- PMCID: PMC3764467
- DOI: 10.1177/1947601913476949
SIRT3: A Central Regulator of Mitochondrial Adaptation in Health and Disease
Abstract
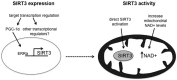
SIRT3 is a NAD(+)-dependent deacetylase that regulates the function of numerous mitochondrial proteins with roles in metabolism, oxidative stress, and cell survival. It is emerging as an instrumental regulator of the mitochondrial adaptive responses to stress, including metabolic reprogramming and enhancing antioxidant defense mechanisms. Here, we discuss the role that SIRT3 plays at both a cellular and physiological level and consider its involvement in disease. Mitochondrial dysfunction is a key contributing factor in many diseases; however, the mechanisms involved are often not well understood, and few targeted therapies exist. If manipulation of SIRT3 proves to be beneficial in disease states, then it could be a promising target for novel therapies.
Keywords: cell protection; mitochondria; sirtuins; stress.
Conflict of interest statement
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
