Engineering E. coli strain for conversion of short chain fatty acids to bioalcohols
- PMID: 24020887
- PMCID: PMC3847231
- DOI: 10.1186/1754-6834-6-128
Engineering E. coli strain for conversion of short chain fatty acids to bioalcohols
Abstract
Background: Recent progress in production of various biofuel precursors and molecules, such as fatty acids, alcohols and alka(e)nes, is a significant step forward for replacing the fossil fuels with renewable fuels. A two-step process, where fatty acids from sugars are produced in the first step and then converted to corresponding biofuel molecules in the second step, seems more viable and attractive at this stage. We have engineered an Escherichia coli strain to take care of the second step for converting short chain fatty acids into corresponding alcohols by using butyrate kinase (Buk), phosphotransbutyrylase (Ptb) and aldehyde/alcohol dehydrogenase (AdhE2) from Clostridium acetobutylicum.
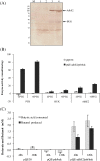
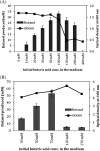
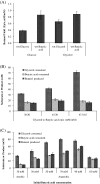
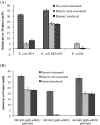
Results: The engineered E. coli was able to convert butyric acid and other short chain fatty acids of chain length C3 to C7 into corresponding alcohols and the efficiency of conversion varied with different E. coli strain type. Glycerol proved to be a better donor of ATP and electron as compared to glucose for converting butyric acid to butanol. The engineered E. coli was able to tolerate up to 100 mM butyric acid and produced butanol with the conversion rate close to 100% under anaerobic condition. Deletion of native genes, such as fumarate reductase (frdA) and alcohol dehydrogenase (adhE), responsible for side products succinate and ethanol, which act as electron sink and could compete with butyric acid uptake, did not improve the butanol production efficiency. Indigenous acyl-CoA synthetase (fadD) was found to play no role in the conversion of butyric acid to butanol. Engineered E. coli was cultivated in a bioreactor under controlled condition where 60 mM butanol was produced within 24 h of cultivation. A continuous bioreactor with the provision of cell recycling allowed the continuous production of butanol at the average productivity of 7.6 mmol/l/h until 240 h.
Conclusions: E. coli engineered with the pathway from C. acetobutylicum could efficiently convert butyric acid to butanol. Other short chain fatty acids with the chain length of C3 to C7 were also converted to the corresponding alcohols. The ability of engineered strain to convert butyric acid to butanol continuously demonstrates commercial significance of the system.
Figures
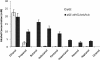

References
-
- Mussatto SI, Dragone G, Guimarães PM, Silva JP, Carneiro LM, Roberto IC, Vicente A, Domingues L, Teixeira JA. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv. 2010;28:817–830. - PubMed
-
- Jin C, Yao M, Liu H, Leed CF, Ji J. Progress in the production and application of n-butanol as a biofuel. Renew Sustain Energy Rev. 2011;15:4080–4106. doi: 10.1016/j.rser.2011.06.001. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

