Preclinical toxicology and biodistribution studies of recombinant adeno-associated virus 1 human acid α-glucosidase
- PMID: 24021025
- PMCID: PMC4003472
- DOI: 10.1089/humc.2013.147
Preclinical toxicology and biodistribution studies of recombinant adeno-associated virus 1 human acid α-glucosidase
Abstract
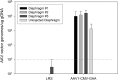
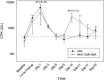
A biodistribution and toxicology study was performed to test the acute toxicities of intradiaphragmatic injection of a recombinant adeno-associated virus (rAAV) 2/1-human acid alpha-Glucosidase (hGAA) driven by a cytomegalovirus (CMV) promoter (rAAV1-CMV-hGAA) in New Zealand white rabbits and in the rodent Pompe disease model by injecting at the right quadriceps. Studies performed using fluoroscopy and AAV2-GFP demonstrated spread upon intradiaphragmatic injection, and the ability of AAV to infect and express acid α-glucosidase (GAA) throughout the diaphragm. For the preclinical study, 10 rabbits (5 male, 5 female) were divided into two groups, vehicle control (Lactated Ringer's) and test article (1.5×10(12) vector genomes [vg] rAAV1-CMV-hGAA), and euthanized on day 21. After direct visualization, the left hemidiaphragm was injected at three locations. There was up to a 2,500-fold increase in circulating anti-AAV1 antibodies directed to the vector capsids. In addition, up to an 18-fold increase in antibodies against the GAA protein was generated. Injection sites maintained up to 1.0×10(5) vg/μg genomic DNA (gDNA), while uninjected sites had up to 1.0×10(4) vg/μg gDNA. Vector DNA was present in blood at 24 hr postinjection at up to 1.0×10(6) vg/μg gDNA, followed by a decrease to 1.0×10(3) vg/μg gDNA at euthanization on day 21. Nominal amounts of vector DNA were present in peripheral organs, including the brain, spinal cord, gonads, and skeletal muscle. Upon histopathological examination, fibroplasias of the serosal surface were noted at diaphragm injections sites of both groups. In addition, an increase in mononuclear cell infiltration in the diaphragm and esophagus in vector-dosed animals was found. Elevated creatine phosphokinase levels, an indicator of muscle repair, was observed in all animals postprocedure but persisted in vector-injected rabbits until euthanization. A follow-up study suggested that this was directed against the human transgene expression in a foreign species. Overall, this study demonstrates diffusion of vector throughout the diaphragm after localized injections.
Figures



References
-
- Brantly M.L. Spencer L.T. Humphries M., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum. Gene. Ther. 2006;17:1177–1186. - PubMed
-
- Chao H. Monahan P.E. Liu Y., et al. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol. Ther. 2001;4:217–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

