Aminoacylation of tRNA 2'- or 3'-hydroxyl by phosphoseryl- and pyrrolysyl-tRNA synthetases
- PMID: 24021645
- PMCID: PMC3830498
- DOI: 10.1016/j.febslet.2013.08.037
Aminoacylation of tRNA 2'- or 3'-hydroxyl by phosphoseryl- and pyrrolysyl-tRNA synthetases
Abstract
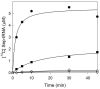
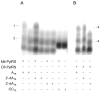
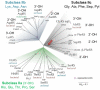
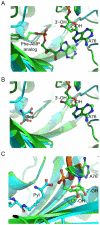
Class I and II aminoacyl-tRNA synthetases (AARSs) attach amino acids to the 2'- and 3'-OH of the tRNA terminal adenosine, respectively. One exception is phenylalanyl-tRNA synthetase (PheRS), which belongs to Class II but attaches phenylalanine to the 2'-OH. Here we show that two Class II AARSs, O-phosphoseryl- (SepRS) and pyrrolysyl-tRNA (PylRS) synthetases, aminoacylate the 2'- and 3'-OH, respectively. Structure-based-phylogenetic analysis reveals that SepRS is more closely related to PheRS than PylRS, suggesting that the idiosyncratic feature of 2'-OH acylation evolved after the split between PheRS and PylRS. Our work completes the understanding of tRNA aminoacylation positions for the 22 natural AARSs.
Keywords: Amino acid; Protein synthesis; tRNA esterification.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Pyrrolysyl-tRNA synthetase variants reveal ancestral aminoacylation function.FEBS Lett. 2013 Oct 1;587(19):3243-8. doi: 10.1016/j.febslet.2013.08.018. Epub 2013 Aug 28. FEBS Lett. 2013. PMID: 23994531 Free PMC article.
-
Recognition of non-alpha-amino substrates by pyrrolysyl-tRNA synthetase.J Mol Biol. 2009 Feb 6;385(5):1352-60. doi: 10.1016/j.jmb.2008.11.059. Epub 2008 Dec 11. J Mol Biol. 2009. PMID: 19100747
-
Characterizing the amino acid activation center of the naturally editing-deficient aminoacyl-tRNA synthetase PheRS in Mycoplasma mobile.FEBS Lett. 2022 Apr;596(7):947-957. doi: 10.1002/1873-3468.14287. Epub 2022 Jan 30. FEBS Lett. 2022. PMID: 35038769
-
Structural analyses of the malaria parasite aminoacyl-tRNA synthetases provide new avenues for antimalarial drug discovery.Protein Sci. 2021 Sep;30(9):1793-1803. doi: 10.1002/pro.4148. Protein Sci. 2021. PMID: 34184352 Free PMC article. Review.
-
Plasticity and Constraints of tRNA Aminoacylation Define Directed Evolution of Aminoacyl-tRNA Synthetases.Int J Mol Sci. 2019 May 9;20(9):2294. doi: 10.3390/ijms20092294. Int J Mol Sci. 2019. PMID: 31075874 Free PMC article. Review.
Cited by
-
Pyrrolysine Aminoacyl-tRNA Synthetase as a Tool for Expanding the Genetic Code.Int J Mol Sci. 2025 Jan 10;26(2):539. doi: 10.3390/ijms26020539. Int J Mol Sci. 2025. PMID: 39859254 Free PMC article. Review.
-
Aminoacyl-tRNA Synthetases in the Bacterial World.EcoSal Plus. 2016 May;7(1):10.1128/ecosalplus.ESP-0002-2016. doi: 10.1128/ecosalplus.ESP-0002-2016. EcoSal Plus. 2016. PMID: 27223819 Free PMC article. Review.
-
System-wide optimization of an orthogonal translation system with enhanced biological tolerance.Mol Syst Biol. 2023 Aug 8;19(8):e10591. doi: 10.15252/msb.202110591. Epub 2023 Jul 21. Mol Syst Biol. 2023. PMID: 37477096 Free PMC article.
-
Single nucleotide translation without ribosomes.Nat Chem. 2021 Aug;13(8):751-757. doi: 10.1038/s41557-021-00749-4. Epub 2021 Jul 26. Nat Chem. 2021. PMID: 34312504
-
Expanding the substrate scope of pyrrolysyl-transfer RNA synthetase enzymes to include non-α-amino acids in vitro and in vivo.Nat Chem. 2023 Jul;15(7):960-971. doi: 10.1038/s41557-023-01224-y. Epub 2023 Jun 1. Nat Chem. 2023. PMID: 37264106 Free PMC article.
References
-
- Ibba M, Söll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. - PubMed
-
- Dale T, Uhlenbeck OC. Amino acid specificity in translation. Trends Biochem Sci. 2005;30:659–665. - PubMed
-
- Steitz TA. A structural understanding of the dynamic ribosome machine. Nat Rev Mol Cell Biol. 2008;9:242–253. - PubMed
-
- Sprinzl M. Chemistry of aminoacylation and peptide bond formation on the 3'terminus of tRNA. J Biosci. 2006;31:489–496. - PubMed
-
- Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

