Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials
- PMID: 24021722
- PMCID: PMC3768250
- DOI: 10.1136/bmj.f3755
Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials
Abstract
Objectives: To evaluate the completeness of descriptions of non-pharmacological interventions in randomised trials, identify which elements are most frequently missing, and assess whether authors can provide missing details.
Design: Analysis of consecutive sample of randomised trials of non-pharmacological interventions.
Data sources and study selection: All reports of randomised trials of non-pharmacological interventions published in 2009 in six leading general medical journals; 133 trial reports, with 137 interventions, met the inclusion criteria.
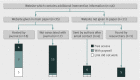
Data collection: Using an eight item checklist, two raters assessed the primary full trial report, plus any reference materials, appendices, or websites. Questions about missing details were emailed to corresponding authors, and relevant items were then reassessed.
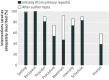
Results: Of 137 interventions, only 53 (39%) were adequately described; this was increased to 81 (59%) by using 63 responses from 88 contacted authors. The most frequently missing item was the "intervention materials" (47% complete), but it also improved the most after author response (92% complete). Whereas some authors (27/70) provided materials or further information, other authors (21/70) could not; their reasons included copyright or intellectual property concerns, not having the materials or intervention details, or being unaware of their importance. Although 46 (34%) trial interventions had further information or materials readily available on a website, many were not mentioned in the report, were not freely accessible, or the URL was no longer functioning.
Conclusions: Missing essential information about interventions is a frequent, yet remediable, contributor to the worldwide waste in research funding. If trial reports do not have a sufficient description of interventions, other researchers cannot build on the findings, and clinicians and patients cannot reliably implement useful interventions. Improvement will require action by funders, researchers, and publishers, aided by long term repositories of materials linked to publications.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Comment in
-
Descriptions of non-pharmacological interventions in clinical trials.BMJ. 2013 Sep 11;347:f5212. doi: 10.1136/bmj.f5212. BMJ. 2013. PMID: 24026465 No abstract available.
References
-
- Aronson JK. Patent medicines and secret remedies. BMJ 2009;339:b5415. - PubMed
-
- Duff JM, Leather H, Walden EO, LaPlant KD, George TJ. Adequacy of published oncology randomized controlled trials to provide therapeutic details needed for clinical application. J Natl Cancer Inst 2010;102:702-5. - PubMed
-
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomised trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-310. - PubMed
-
- Davidson KW, Goldstein M, Kaplan RM, Kaufmann PG, Knatterud GL, Orleans CT, et al. Evidence-based behavioral medicine: what is it and how do we achieve it? Ann Behav Med 2003;26:161-71. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
