UV-B-induced DNA damage and repair in the mouse lens
- PMID: 24022010
- PMCID: PMC3799563
- DOI: 10.1167/iovs.13-12644
UV-B-induced DNA damage and repair in the mouse lens
Abstract
Purpose: Epidemiologic studies have linked UV-B exposure to development of cortical cataracts, but the underlying molecular mechanism(s) is unresolved. Here, we used a mouse model to examine the nature and distribution of DNA photolesions produced by ocular UV-B irradiation.
Methods: Anesthetized mice, eye globes, or isolated lenses were exposed to UV-B. Antibodies specific for 6-4 photoproducts (6-4 PPs) or cyclobutane pyrimidine dimers (CPDs) were used to visualize DNA adducts.
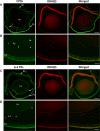
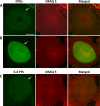
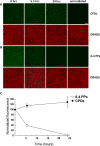
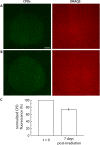
Results: Illumination of intact globes with UV-B-induced 6-4 PP and CPD formation in cells of the cornea, anterior iris, and central lens epithelium. Photolesions were not detected in retina or lens cells situated in the shadow of the iris. Photolesions in lens epithelial cells were produced with radiant exposures significantly below the minimal erythemal dose. Lens epithelial cells rapidly repaired 6-4 PPs, but CPD levels did not markedly diminish, even over extended postirradiation recovery periods in vitro or in vivo. The repair of 6-4 PPs did not depend on the proliferative activity of the epithelial cells, since the repair rate in the mitotically-active germinative zone (GZ) was indistinguishable from that of quiescent cells in the central epithelium.
Conclusions: Even relatively modest exposures to UV-B produced 6-4 PP and CPD photolesions in lens epithelial cells. Cyclobutane pyrimidine dimer lesions were particularly prevalent and were repaired slowly if at all. Studies on sun-exposed skin have established a causal connection between photolesions and so-called UV-signature mutations. If similar mechanisms apply in the lens, it suggests that somatic mutations in lens epithelial cells may contribute to the development of cortical cataracts.
Keywords: DNA damage; UV-B; cortical cataract.
Figures



Similar articles
-
Mitotic Activation Around Wound Edges and Epithelialization Repair in UVB-Induced Capsular Cataracts.Invest Ophthalmol Vis Sci. 2021 Dec 1;62(15):29. doi: 10.1167/iovs.62.15.29. Invest Ophthalmol Vis Sci. 2021. PMID: 34967856 Free PMC article.
-
Ultrastructure of UVR-B-induced cataract and repair visualized with electron microscopy.Acta Ophthalmol. 2014 Nov;92(7):635-43. doi: 10.1111/aos.12376. Epub 2014 Mar 25. Acta Ophthalmol. 2014. PMID: 24666994
-
Ultraviolet light induced DNA damage and repair in bovine lens epithelial cells.Curr Eye Res. 1990 Dec;9(12):1185-93. doi: 10.3109/02713689009003475. Curr Eye Res. 1990. PMID: 2091898
-
UV damage to the eye lens: further results from animal model studies: a review.J Epidemiol. 1999 Dec;9(6 Suppl):S39-47. doi: 10.2188/jea.9.6sup_39. J Epidemiol. 1999. PMID: 10709349 Review.
-
A review of the evidence that ultraviolet irradiation is a risk factor in cataractogenesis.Doc Ophthalmol. 1994-1995;88(3-4):205-20. doi: 10.1007/BF01203675. Doc Ophthalmol. 1994. PMID: 7634990 Review.
Cited by
-
Mitotic Activation Around Wound Edges and Epithelialization Repair in UVB-Induced Capsular Cataracts.Invest Ophthalmol Vis Sci. 2021 Dec 1;62(15):29. doi: 10.1167/iovs.62.15.29. Invest Ophthalmol Vis Sci. 2021. PMID: 34967856 Free PMC article.
-
Phototoxicity of environmental radiations in human lens: revisiting the pathogenesis of UV-induced cataract.Graefes Arch Clin Exp Ophthalmol. 2019 Oct;257(10):2065-2077. doi: 10.1007/s00417-019-04390-3. Epub 2019 Jun 21. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 31227898 Review.
-
Protein expression of nucleolar protein 12 in the retina and its implication in protection of retina from UV irradiation damage.Cell Death Discov. 2024 Mar 11;10(1):130. doi: 10.1038/s41420-024-01902-x. Cell Death Discov. 2024. PMID: 38467618 Free PMC article.
-
TiO2-Nanoparticle-Enhanced Sonodynamic Therapy for Prevention of Posterior Capsular Opacification and Ferroptosis Exploration of Its Mechanism.Invest Ophthalmol Vis Sci. 2024 Oct 1;65(12):24. doi: 10.1167/iovs.65.12.24. Invest Ophthalmol Vis Sci. 2024. PMID: 39417751 Free PMC article.
-
Effects of ELL-associated factor 2 on ultraviolet radiation-induced cataract formation in mice.Mol Med Rep. 2015 Nov;12(5):6605-11. doi: 10.3892/mmr.2015.4281. Epub 2015 Sep 2. Mol Med Rep. 2015. PMID: 26328919 Free PMC article.
References
-
- Kopp G, Lean JL. A new, lower value of total solar irradiance: evidence and climate significance. Geophys Res Lett. 2011; 38 10.1029/2010GL045777 - DOI
-
- Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002; 28: 4–13 - PubMed
-
- Sliney DH. Intraocular and crystalline lens protection from ultraviolet damage. Eye Contact Lens. 2011; 37: 250–258 - PubMed
-
- Lucas R, McMichael T, Smith W, Armstrong B. Solar Ultraviolet Radiation: Global Burden of Disease From Solar Ultraviolet Radiation. Geneva: World Health Organization; 2006.
-
- Beukers R, Eker AP, Lohman PH. 50 years thymine dimer. DNA Repair (Amst). 2008; 7: 530–543 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

