Aberrant muscle antigen exposure in mice is sufficient to cause myositis in a Treg cell-deficient milieu
- PMID: 24022275
- PMCID: PMC4033530
- DOI: 10.1002/art.38184
Aberrant muscle antigen exposure in mice is sufficient to cause myositis in a Treg cell-deficient milieu
Abstract
Objective: Myositis is associated with muscle-targeted inflammation and is observed in some Treg cell-deficient mouse models. Because an autoimmune pathogenesis has been strongly implicated, the aim of this study was to investigate the hypothesis that abnormal exposure to muscle antigens, as observed in muscle injury, can induce autoimmune-mediated myositis in susceptible hosts.
Methods: FoxP3 mutant (scurfy) mice were mated to synaptotagmin VII (Syt VII) mutant mice, which resulted in a new mouse strain that combines impaired membrane resealing with Treg cell deficiency. Lymphocyte preparations from double-mutant mice were adoptively transferred intraperitoneally, with or without purified Treg cells, into recombination-activating gene 1 (RAG-1)-null recipients. Lymph node cells from mice with the FoxP3 mutation were transferred into RAG-1-null mice either 1) intraperitoneally in conjunction with muscle homogenate or purified myosin protein or 2) intramuscularly with or without cotransfer of purified Treg cells.
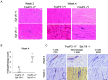
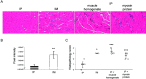
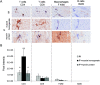
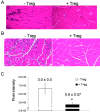
Results: FoxP3-deficient mouse lymph node cells transferred in conjunction with myosin protein or muscle homogenate induced robust skeletal muscle inflammation. The infiltrates consisted predominantly of CD4+ and CD8+ T cells, a limited number of macrophages, and no B cells. Significant inflammation was also seen in similar experiments using lymph node cells from FoxP3/Syt VII double-mutant mice but was absent in experiments using adoptive transfer of FoxP3 mutant mouse cells alone. The cotransfer of Treg cells completely suppressed myositis.
Conclusion: These data, derived from a new, reproducible model, demonstrate the critical roles of Treg cell deficiency and aberrant muscle antigen exposure in the priming of autoreactive cells to induce myositis. This mouse system has multifaceted potential for examining the interplay in vivo between tissue injury and autoimmunity.
© 2013 The Authors. Arthritis & Rheumatism is published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology.
Figures


Comment in
-
Inflammatory myopathies: T(REG)-cell deficiency and abnormal muscle antigen exposure important to the development of myositis.Nat Rev Rheumatol. 2013 Nov;9(11):638. doi: 10.1038/nrrheum.2013.149. Epub 2013 Oct 1. Nat Rev Rheumatol. 2013. PMID: 24080863 No abstract available.
Similar articles
-
Role of regulatory T cells in a new mouse model of experimental autoimmune myositis.Am J Pathol. 2009 Mar;174(3):989-98. doi: 10.2353/ajpath.2009.080422. Epub 2009 Feb 13. Am J Pathol. 2009. PMID: 19218348 Free PMC article.
-
Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25- regulatory T cells.J Immunol. 2006 Apr 15;176(8):4748-56. doi: 10.4049/jimmunol.176.8.4748. J Immunol. 2006. PMID: 16585568
-
Large functional repertoire of regulatory T-cell suppressible autoimmune T cells in scurfy mice.J Autoimmun. 2007 Aug;29(1):10-9. doi: 10.1016/j.jaut.2007.04.001. Epub 2007 May 23. J Autoimmun. 2007. PMID: 17521882 Free PMC article.
-
The role of FOXP3+ regulatory T cells in human autoimmune and inflammatory diseases.Clin Exp Immunol. 2019 Jul;197(1):24-35. doi: 10.1111/cei.13288. Epub 2019 Mar 24. Clin Exp Immunol. 2019. PMID: 30830965 Free PMC article. Review.
-
Regulation of multi-organ inflammation in the regulatory T cell-deficient scurfy mice.J Biomed Sci. 2009 Feb 12;16(1):20. doi: 10.1186/1423-0127-16-20. J Biomed Sci. 2009. PMID: 19272184 Free PMC article. Review.
Cited by
-
Aromatase-Inhibitor-Induced Musculoskeletal Inflammation Is Observed Independent of Oophorectomy in a Novel Mouse Model.Pharmaceuticals (Basel). 2022 Dec 17;15(12):1578. doi: 10.3390/ph15121578. Pharmaceuticals (Basel). 2022. PMID: 36559029 Free PMC article.
-
Inhibition of miRNA associated with a disease-specific signature and secreted via extracellular vesicles of systemic lupus erythematosus patients suppresses target organ inflammation in a humanized mouse model.Front Immunol. 2024 Jun 13;14:1090177. doi: 10.3389/fimmu.2023.1090177. eCollection 2023. Front Immunol. 2024. PMID: 38939646 Free PMC article.
-
Inflammatory myopathies: T(REG)-cell deficiency and abnormal muscle antigen exposure important to the development of myositis.Nat Rev Rheumatol. 2013 Nov;9(11):638. doi: 10.1038/nrrheum.2013.149. Epub 2013 Oct 1. Nat Rev Rheumatol. 2013. PMID: 24080863 No abstract available.
-
Daily Moderate Exercise Is Beneficial and Social Stress Is Detrimental to Disease Pathology in Murine Lupus Nephritis.Front Physiol. 2017 Apr 26;8:236. doi: 10.3389/fphys.2017.00236. eCollection 2017. Front Physiol. 2017. PMID: 28491039 Free PMC article.
-
Targeting Tregs in Juvenile Idiopathic Arthritis and Juvenile Dermatomyositis-Insights From Other Diseases.Front Immunol. 2019 Jan 25;10:46. doi: 10.3389/fimmu.2019.00046. eCollection 2019. Front Immunol. 2019. PMID: 30740105 Free PMC article. Review.
References
-
- Figarella-Branger D, Civatte M, Bartoli C, Pellissier JF. Cytokines, chemokines, and cell adhesion molecules in inflammatory myopathies. Muscle Nerve. 2003;28:659–82. - PubMed
-
- Christopher-Stine L, Plotz PH. Myositis: an update on pathogenesis. Curr Opin Rheumatol. 2004;16:700–6. - PubMed
-
- Sontheimer RD. The management of dermatomyositis: current treatment options. Expert Opin Pharmacother. 2004;5:1083–99. - PubMed
-
- Greenberg SA. Inflammatory myopathies: evaluation and management. Semin Neurol. 2008;28:241–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

