Opposing actions of environmental enrichment and Alzheimer's disease on the expression of hippocampal microRNAs in mouse models
- PMID: 24022509
- PMCID: PMC3784766
- DOI: 10.1038/tp.2013.77
Opposing actions of environmental enrichment and Alzheimer's disease on the expression of hippocampal microRNAs in mouse models
Abstract
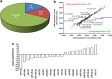
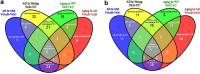
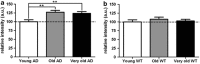
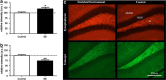
Alzheimer's disease (AD) is the most common form of dementia in the elderly. Although there are no drugs that modify the disease process, exposure to an enriched environment (EE) can slow the disease progression. Here, we characterize the effects of AD and EE on the post-transcriptional regulators, microRNAs (miRNAs), which may contribute to the detrimental and beneficial effects of AD and EE, respectively, on synaptic plasticity-related proteins and AD pathology. We found for the first time miRNAs that were inversely regulated in AD and EE, and may affect synaptic proteins and modulators, molecular factors associated with AD pathology, and survival and neuroprotective factors. MiRNAs that were upregulated only in 3xTgAD mice model of AD compared with their control mice were localized to synapses, predicted to downregulate essential synaptic proteins and are highly associated with regulating apoptosis, AD-associated processes and axon guidance. Studying the progressive change in miRNAs modulation during aging of 3xTgAD mice, we identified miRNAs that were regulated in earlier stages of AD, suggesting them as potential AD biomarkers. Last, we characterized AD- and EE-related effects in the mouse hippocampus on tomosyn protein levels, an inhibitor of the synaptic transmission machinery. While EE reduced tomosyn levels, tomosyn levels were increased in old 3xTgAD mice, suggesting a role for tomosyn in the impairment of synaptic transmission in AD. Interestingly, we found that miR-325 regulates the expression levels of tomosyn as demonstrated by a luciferase reporter assay, and that miR-325 was downregulated in AD and upregulated following EE. These findings improve our understanding of the molecular and cellular processes in AD pathology, following EE, and the interplay between the two processes, and open new avenues for the studies of understanding and controlling AD.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

