Activation of the ubiquitin proteasome pathway in a mouse model of inflammatory myopathy: a potential therapeutic target
- PMID: 24022788
- PMCID: PMC4080828
- DOI: 10.1002/art.38180
Activation of the ubiquitin proteasome pathway in a mouse model of inflammatory myopathy: a potential therapeutic target
Abstract
Objective: Myositis is characterized by severe muscle weakness. We and others have previously shown that endoplasmic reticulum (ER) stress plays a role in the pathogenesis of myositis. The present study was undertaken to identify perturbed pathways and assess their contribution to muscle disease in a mouse myositis model.
Methods: Stable isotope labeling with amino acids in cell culture (SILAC) was used to identify alterations in the skeletal muscle proteome of myositic mice in vivo. Differentially altered protein levels identified in the initial comparisons were validated using a liquid chromatography tandem mass spectrometry spike-in strategy and further confirmed by immunoblotting. In addition, we evaluated the effect of a proteasome inhibitor, bortezomib, on the disease phenotype, using well-standardized functional, histologic, and biochemical assessments.
Results: With the SILAC technique we identified significant alterations in levels of proteins belonging to the ER stress response, ubiquitin proteasome pathway (UPP), oxidative phosphorylation, glycolysis, cytoskeleton, and muscle contractile apparatus categories. We validated the myositis-related changes in the UPP and demonstrated a significant increase in the ubiquitination of muscle proteins as well as a specific increase in ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCHL-1) in myositis, but not in muscle affected by other dystrophies or normal muscle. Inhibition of the UPP with bortezomib significantly improved muscle function and also significantly reduced tumor necrosis factor α expression in the skeletal muscle of mice with myositis.
Conclusion: Our findings indicate that ER stress activates downstream UPPs and contributes to muscle degeneration and that UCHL-1 is a potential biomarker for disease progression. UPP inhibition offers a potential therapeutic strategy for myositis.
Copyright © 2013 by the American College of Rheumatology.
Figures
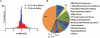
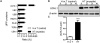
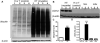
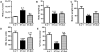
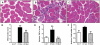

Similar articles
-
Proteasome inhibition in skeletal muscle cells unmasks metabolic derangements in type 2 diabetes.Am J Physiol Cell Physiol. 2014 Nov 1;307(9):C774-87. doi: 10.1152/ajpcell.00110.2014. Epub 2014 Aug 20. Am J Physiol Cell Physiol. 2014. PMID: 25143351
-
Therapeutic landscape of carfilzomib and other modulators of the ubiquitin-proteasome pathway.J Clin Oncol. 2015 Mar 1;33(7):782-5. doi: 10.1200/JCO.2014.55.5748. Epub 2015 Jan 20. J Clin Oncol. 2015. PMID: 25605842 Free PMC article. No abstract available.
-
The ubiquitin-proteasome system regulates the stability of neuronal nicotinic acetylcholine receptors.J Mol Neurosci. 2010 Jan;40(1-2):177-84. doi: 10.1007/s12031-009-9272-x. Epub 2009 Aug 20. J Mol Neurosci. 2010. PMID: 19693707 Free PMC article.
-
Advances in the understanding of mechanisms and therapeutic use of bortezomib.Discov Med. 2011 Dec;12(67):471-80. Discov Med. 2011. PMID: 22204764 Free PMC article. Review.
-
When ubiquitin meets NF-κB: a trove for anti-cancer drug development.Curr Pharm Des. 2013;19(18):3263-75. doi: 10.2174/1381612811319180010. Curr Pharm Des. 2013. PMID: 23151140 Free PMC article. Review.
Cited by
-
Unraveling the molecular heterogeneity in type 2 diabetes: a potential subtype discovery followed by metabolic modeling.BMC Med Genomics. 2020 Aug 24;13(1):119. doi: 10.1186/s12920-020-00767-0. BMC Med Genomics. 2020. PMID: 32831068 Free PMC article.
-
Gene Expression Meta-Analysis Reveals Concordance in Gene Activation, Pathway, and Cell-Type Enrichment in Dermatomyositis Target Tissues.ACR Open Rheumatol. 2019 Nov 9;1(10):657-666. doi: 10.1002/acr2.11081. eCollection 2019 Dec. ACR Open Rheumatol. 2019. PMID: 31872188 Free PMC article.
-
Idiopathic inflammatory myopathy human derived cells retain their ability to increase mitochondrial function.PLoS One. 2020 Nov 20;15(11):e0242443. doi: 10.1371/journal.pone.0242443. eCollection 2020. PLoS One. 2020. PMID: 33216776 Free PMC article.
-
Proteomic Profiling of Hindlimb Skeletal Muscle Disuse in a Murine Model of Sepsis.Crit Care Explor. 2024 Aug 20;6(8):e1144. doi: 10.1097/CCE.0000000000001144. eCollection 2024 Aug 1. Crit Care Explor. 2024. PMID: 39162648 Free PMC article.
-
Role of Toll-like receptors in the pathogenesis of dystrophin-deficient skeletal and heart muscle.Hum Mol Genet. 2014 May 15;23(10):2604-17. doi: 10.1093/hmg/ddt656. Epub 2013 Dec 23. Hum Mol Genet. 2014. PMID: 24368419 Free PMC article.
References
-
- Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52:1824–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR050478/AR/NIAMS NIH HHS/United States
- K26 OD011171/OD/NIH HHS/United States
- K26-OD-011171/OD/NIH HHS/United States
- 2R24-HD-050846-06/HD/NICHD NIH HHS/United States
- P30 HD040677/HD/NICHD NIH HHS/United States
- P30-HD-40677/HD/NICHD NIH HHS/United States
- 5U54-HD-053177/HD/NICHD NIH HHS/United States
- R24 HD050846/HD/NICHD NIH HHS/United States
- R01-AR-050478/AR/NIAMS NIH HHS/United States
- 5P30-HD-040677-10/HD/NICHD NIH HHS/United States
- UL1-RR-031988/RR/NCRR NIH HHS/United States
- U54 HD053177/HD/NICHD NIH HHS/United States
- UL1 RR031988/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

