Generation and characterization of a diabody targeting the αvβ6 integrin
- PMID: 24023846
- PMCID: PMC3762766
- DOI: 10.1371/journal.pone.0073260
Generation and characterization of a diabody targeting the αvβ6 integrin
Abstract
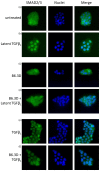
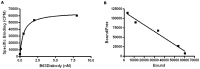
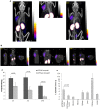
The αvβ6 integrin is up-regulated in cancer and wound healing but it is not generally expressed in healthy adult tissue. There is increasing evidence that it has a role in cancer progression and will be a useful target for antibody-directed cancer therapies. We report a novel recombinant diabody antibody fragment that targets specifically αvβ6 and blocks its function. The diabody was engineered with a C-terminal hexahistidine tag (His tag), expressed in Pichia pastoris and purified by IMAC. Surface plasmon resonance (SPR) analysis of the purified diabody showed affinity in the nanomolar range. Pre-treatment of αvβ6-expressing cells with the diabody resulted in a reduction of cell migration and adhesion to LAP, demonstrating biological function-blocking activity. After radio-labeling, using the His-tag for site-specific attachment of (99m)Tc, the diabody retained affinity and targeted specifically to αvβ6-expressing tumors in mice bearing isogenic αvβ6 +/- xenografts. Furthermore, the diabody was specifically internalized into αvβ6-expressing cells, indicating warhead targeting potential. Our results indicate that the new αvβ6 diabody has a range of potential applications in imaging, function blocking or targeted delivery/internalization of therapeutic agents.
Conflict of interest statement
Figures


References
-
- Patsenker E, Wilkens L, Banz V, Osterreicher CH, Weimann R, et al. (2010) The alphavbeta6 integrin is a highly specific immunohistochemical marker for cholangiocarcinoma. J Hepatol 52: 362–369. - PubMed
-
- Sipos B, Hahn D, Carceller A, Piulats J, Hedderich J, et al. (2004) Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology 45: 226–236. - PubMed
-
- Bates RC (2005) Colorectal cancer progression: integrin alphavbeta6 and the epithelial-mesenchymal transition (EMT). Cell Cycle 4: 1350–1352. - PubMed
-
- Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, et al. (2007) Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol 212: 316–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

