Expression of Livin in colorectal cancer and its relationship to tumor cell behavior and prognosis
- PMID: 24023847
- PMCID: PMC3759411
- DOI: 10.1371/journal.pone.0073262
Expression of Livin in colorectal cancer and its relationship to tumor cell behavior and prognosis
Abstract
Backgrounds: Expression of Livin, a member of the inhibitors of apoptosis protein family, is associated with tumor development and progression. The aims of this study were to evaluate whether Livin affects oncogenic biological behavior of colorectal cancer cells, and to document the relationship between its expression and various clinicopathological parameters in colorectal cancer.
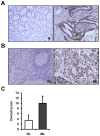
Methods: We investigated the impact of Livin on tumor cell behavior by using the small interfering RNA and pcDNA3.1 vector in SW480 and DKO1 colorectal cancer cell lines. The expression of Livin was investigated by RT-PCR and immunohistochemistry in coloretcal cancer tissues. The apoptotic cells were visualized by TUNEL assay, and proliferative cells were visualized by Ki-67 antibody staining.
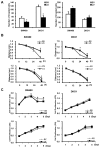
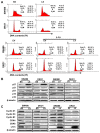
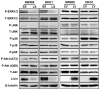
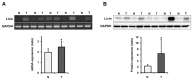
Results: Knockdown of Livin suppressed tumor cell migration and invasion in colorectal cancer cells. Knockdown of Livin induced the apoptosis by up-regulating of caspase-3, -7 and PARP activities and the cell cycle arrest by decreasing cyclin D1, cyclin D3, cyclin-dependent kinase 4 and 6, and by inducing p27 expression. The MAPK signaling cascades were significantly blocked by knockdown of Livin. In contrast, overexpression of Livin enhanced tumor cell migration and invasion, and inhibited the apoptosis and cell cycle arrest. The mean apoptotic index (AI) value of Livin positive tumors was significantly lower than AI of Livin negative tumors. However, there was no significant difference between Livin expression and Ki-67 labeling index (KI). Livin expression was significantly increased in colorectal cancer and metastatic lymph node tissues compared to normal colorectal mucosa and non-metastatic lymph node tissues and was associated with tumor stage, lymphovascular invasion, lymph node metastasis and poor survival.
Conclusions: These results indicate that Livin is associated with tumor progression by increasing tumor cell motility and inhibiting apoptosis in colorectal cancer.
Conflict of interest statement
Figures



References
-
- Ahmed FE (2005) Development of novel diagnostic and prognostic molecular markers for sporadic colon cancer. Expert Rev Mol Diagn 5: 337-352. doi:10.1586/14737159.5.3.337. PubMed: 15934812. - DOI - PubMed
-
- Allen WL, Johnston PG (2005) Have we made progress in pharmacogenomics? The implementation of molecular markers in colon cancer. Pharmacogenomics 6: 603-614. doi:10.2217/14622416.6.6.603. PubMed: 16143000. - DOI - PubMed
-
- Riethdorf S, Wikman H, Pantel K (2008) Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer 123: 1991-2006. doi:10.1002/ijc.23825. PubMed: 18712708. - DOI - PubMed
-
- Jankowski JA, Odze RD (2009) Biomarkers in gastroenterology: between hope and hype comes histopathology. Am J Gastroenterol 104: 1093-1096. doi:10.1038/ajg.2008.172. PubMed: 19417749. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

