The HDAC inhibitor LBH589 induces ERK-dependent prometaphase arrest in prostate cancer via HDAC6 inactivation and down-regulation
- PMID: 24023871
- PMCID: PMC3762759
- DOI: 10.1371/journal.pone.0073401
The HDAC inhibitor LBH589 induces ERK-dependent prometaphase arrest in prostate cancer via HDAC6 inactivation and down-regulation
Abstract
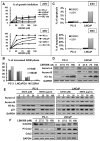
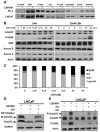
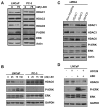
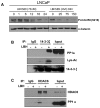
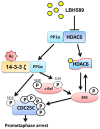
Histone deacetylase inhibitors (HDACIs) have potent anti-cancer activity in a variety of cancer models. Understanding the molecular mechanisms involved in the therapeutic responsiveness of HDACI is needed before its clinical application. This study aimed to determine if a potent HDACI, LBH589 (Panobinostat), had differential therapeutic responsiveness towards LNCaP and PC-3 prostate cancer (PCa) cells. The former showed prometaphase arrest with subsequent apoptosis upon LBH589 treatment, while the latter was less sensitive and had late G2 arrest. The LBH589 treatment down-regulated HDAC6 and sustained ERK activation, and contributed to prometaphase arrest. Mechanistically, LBH589 inhibited HDAC6 activity, caused its dissociation from protein phosphatase PP1α, and increased 14-3-3ζ acetylation. Acetylated 14-3-3ζ released its mask effect on serine 259 of c-Raf and serine 216 of Cdc25C subsequent to de-phosphorylation by PP1α, which contributed to ERK activation. Enhanced ERK activity by LBH589 further down-regulated HDAC6 protein levels and sustained ERK activation by free-forward regulation. The sustained Cdc25C and ERK activation resulted in early M-phase (prometaphase) arrest and subsequent apoptosis in the most sensitive LNCaP cells but not in PC-3 cells. This study provides pre-clinical evidence that HDAC6 may serve as a sensitive therapeutic target in the treatment of prostate cancer with HDACI LBH589 for clinical translation. This study also posits a novel mechanism of HDAC6 participation in regulating the c-Raf-PP1-ERK signaling pathway and contributing to M phase cell-cycle transition.
Conflict of interest statement
Figures


References
-
- Tan J, Cang S, Ma Y, Petrillo RL, Liu D (2010) Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. Hematol Oncol 3: 5. doi:10.1186/1756-8722-3-5. PubMed: 20132536. - DOI - PMC - PubMed
-
- Yang Y, Rao R, Shen J, Tang Y, Fiskus W et al. (2008) Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Res 68: 4833-4842. doi:10.1158/0008-5472.CAN-08-0644. PubMed: 18559531. - DOI - PMC - PubMed
-
- Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38-51. doi:10.1038/nrc1779. PubMed: 16397526. - DOI - PubMed
-
- Richon VM, Sandhoff TW, Rifkind RA, Marks PA (2000) Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A 97: 10014-10019. doi:10.1073/pnas.180316197. PubMed: 10954755. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

