Passive carriage of rabies virus by dendritic cells
- PMID: 24024103
- PMCID: PMC3765594
- DOI: 10.1186/2193-1801-2-419
Passive carriage of rabies virus by dendritic cells
Abstract
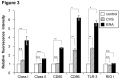
The rabies virus (RABV) is highly neurotropic and it uses evasive strategies to successfully evade the host immune system. Because rabies is often fatal, understanding the basic processes of the virus-host interactions, particularly in the initial events of infection, is critical for the design of new therapeutic approaches to target RABV. Here, we examined the possible role of dendritic cells (DCs) in the transmission of RABV to neural cells at peripheral site of exposure. Viral replication only occurred at a low level in the DC cell line, JAWS II, after its infection with either pathogenic RABV (CVS strain) or low-pathogenic RABV (ERA strain), and no progeny viruses were produced in the culture supernatants. However, both viral genomic RNAs were retained in the long term after infection and maintained their infectivity. The biggest difference between CVS and ERA was in their ability to induce type I interferons. Although the ERA-infected JAWS II cells exhibited cytopathic effect and were apparently killed by normal spleen cells in vitro, the CVS-infected JAWS II cells showed milder cytopathic effect and less lysis when cocultured with spleen cells. Strongly increased expression of major histocompatibility complex classes I, costimulatory molecules (CD80 and CD86), type I interferons and Toll- like receptor 3, and was observed only in the ERA-inoculated JAWS II cells and not in those inoculated with CVS. During the silencing of the cellular immune response in the DCs, the pathogenic CVS strain cryptically maintained an infectious viral genome and was capable of transmitting infectious RABV to permissive neural cells. These findings demonstrate that DCs may play a role in the passive carriage of RABV during natural rabies infections.
Keywords: Dendritic cells; Immune invasion; Rabies virus.
Figures

Similar articles
-
Subversion of the Immune Response by Rabies Virus.Viruses. 2016 Aug 19;8(8):231. doi: 10.3390/v8080231. Viruses. 2016. PMID: 27548204 Free PMC article. Review.
-
Comprehensive Analysis of Protein Acetylation and Glucose Metabolism in Mouse Brains Infected with Rabies Virus.J Virol. 2022 Feb 23;96(4):e0194221. doi: 10.1128/JVI.01942-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878915 Free PMC article.
-
Hydrogel-based 3D human iPSC-derived neuronal culture for the study of rabies virus infection.Front Cell Infect Microbiol. 2023 Aug 25;13:1215205. doi: 10.3389/fcimb.2023.1215205. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37692167 Free PMC article.
-
The inability of wild-type rabies virus to activate dendritic cells is dependent on the glycoprotein and correlates with its low level of the de novo-synthesized leader RNA.J Virol. 2015 Feb;89(4):2157-69. doi: 10.1128/JVI.02092-14. Epub 2014 Dec 3. J Virol. 2015. PMID: 25473057 Free PMC article.
-
Evasive strategies in rabies virus infection.Adv Virus Res. 2011;79:33-53. doi: 10.1016/B978-0-12-387040-7.00003-2. Adv Virus Res. 2011. PMID: 21601041 Review.
Cited by
-
The Immune Escape Strategy of Rabies Virus and Its Pathogenicity Mechanisms.Viruses. 2024 Nov 14;16(11):1774. doi: 10.3390/v16111774. Viruses. 2024. PMID: 39599888 Free PMC article. Review.
-
Subversion of the Immune Response by Rabies Virus.Viruses. 2016 Aug 19;8(8):231. doi: 10.3390/v8080231. Viruses. 2016. PMID: 27548204 Free PMC article. Review.
-
A comparative analysis of the dendritic cell response upon exposure to different rabies virus strains.PLoS Negl Trop Dis. 2025 Apr 10;19(4):e0012994. doi: 10.1371/journal.pntd.0012994. eCollection 2025 Apr. PLoS Negl Trop Dis. 2025. PMID: 40208887 Free PMC article.
-
Human rabies: a descriptive observation of 21 children in Kinshasa, the Democratic Republic of Congo.Pathog Glob Health. 2014 Oct;108(7):317-22. doi: 10.1179/2047773214Y.0000000161. Pathog Glob Health. 2014. PMID: 25417907 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

