Acrolein-induced activation of mitogen-activated protein kinase signaling is mediated by alkylation of thioredoxin reductase and thioredoxin 1
- PMID: 24024160
- PMCID: PMC3757691
- DOI: 10.1016/j.redox.2013.02.001
Acrolein-induced activation of mitogen-activated protein kinase signaling is mediated by alkylation of thioredoxin reductase and thioredoxin 1
Abstract
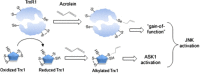
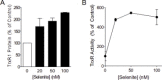
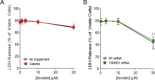
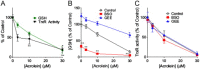
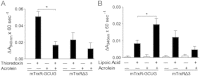
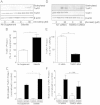
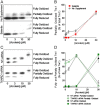
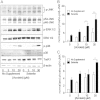
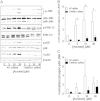
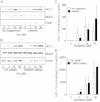
Cigarette smoking remains a major health concern worldwide, and many of the adverse effects of cigarette smoke (CS) can be attributed to its abundant electrophilic aldehydes, such as acrolein (2-propenal). Previous studies indicate that acrolein readily reacts with thioredoxin reductase 1 (TrxR1), a critical enzyme involved in regulation of thioredoxin (Trx)-mediated redox signaling, by alkylation at its selenocysteine (Sec) residue. Because alkylation of Sec within TrxR1 has significant implications for its enzymatic function, we explored the potential importance of TrxR1 alkylation in acrolein-induced activation or injury of bronchial epithelial cells. Exposure of human bronchial epithelial HBE1 cells to acrolein (1-30 μM) resulted in dose-dependent loss of TrxR thioredoxin reductase activity, which coincided with its alkylation, as determined by biotin hydrazide labeling, and was independent of initial GSH status. To test the involvement of TrxR1 in acrolein responses in HBE1 cells, we suppressed TrxR1 using siRNA silencing or augmented TrxR1 by cell supplementation with sodium selenite. Acrolein exposure of HBE1 cells induced dose-dependent activation of the MAP kinases, extracellular regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38, and activation of JNK was markedly enhanced after selenite-mediated induction of TrxR1, and was associated with increased alkylation of TrxR1. Conversely, siRNA silencing of TrxR1 significantly suppressed the ability of acrolein to activate JNK, and also appeared to attenuate acrolein-dependent activation of ERK and p38. Alteration of initial TrxR1 levels by siRNA or selenite supplementation also affected initial Trx1 redox status and acrolein-mediated alkylation of Trx1, but did not significantly affect acrolein-mediated activation of HO-1 or cytotoxicity. Collectively, our findings indicate that alkylation of TrxR1 and/or Trx1 may contribute directly to acrolein-mediated activation of MAP kinases such as JNK, and may therefore be important in acrolein-induced alterations in airway epithelial function, as a contributing mechanism in tobacco-related respiratory disease.
Keywords: Cigarette smoke; Electrophile; Epithelium; GSH; Michael addition; c-Jun N-terminal kinase.
Figures



References
-
- Office of the Surgeon General. The Health Consequences of Smoking: A Report of the Surgeon General; 2004. - PubMed
-
- Saetta M. Airway inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1999;160:S17–20. - PubMed
-
- Thomson N.C., Chaudhuri R. Asthma in smokers: challenges and opportunities. Current Opinion in Pulmonary Medicine. 2009;15:39–45. - PubMed
-
- Heijink I.H., Brandenburg S.M., Postma D.S., van Oosterhout A.J. Cigarette smoke impairs airway epithelial barrier function and cell–cell contact recovery. European Respiratory Journal. 2012;39:419–428. - PubMed
-
- Xiao C., Puddicombe S.M., Field S., Haywood J., Broughton-Head V., Puxeddu I., Haitchi H.M., Vernon-Wilson E., Sammut D., Bedke N., Cremin C., Sones J., Djukanovic R., Howarth P.H., Collins J.E., Holgate S.T., Monk P., Davies D.E. Defective epithelial barrier function in asthma. Journal of Allergy and Clinical Immunology. 2011;128:549–556. e541-512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

