Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy
- PMID: 24024169
- PMCID: PMC3757703
- DOI: 10.1016/j.redox.2013.05.003
Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy
Abstract
Objective: Ovarian senescence affects many tissues and produces a variety of symptoms and signs. We hypothesized that estrogens may also influence circulating redox balance by regulating activity of the cellular antioxidative enzyme system. We aimed to explore the impact of surgical estrogen deprivation and replacement (ERT) on the glutathione balance and antioxidant enzymes expression in fertile women.
Study design: Nineteen healthy premenopausal women who underwent total hysterectomy with bilateral salpingo-oophorectomy were evaluated at baseline, 30 days after surgery without ERT and 30 days after ERT. Redox balance was determined by measuring blood reduced (GSH) and oxidized (GSSG) glutathione, as well as the GSSG/GSH ratio. Antioxidant status was evaluated by measuring serum estrogen (E2) levels and mRNA expression of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione S-transferase (GST) in peripheral blood mononuclear cells.
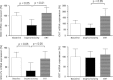
Results: Serum E2 significantly lowered after surgery, and increased in 12 out of 19 patients after 30 days of ERT (Responders). In such patients, an increase in oxidative stress was observed after surgery that resolved after ERT. Oxidative stress was sustained by reduction in the mRNA expression of both SOD and GSH-Px, that recovered after 30 days of therapy in responders. CAT and GST mRNA expression were not modified by surgery and replacement therapy.
Conclusions: Menopause is associated with significant change in antioxidant gene expression that in turn affects circulating redox state. Estrogens replacement therapy is able to prevent and counteract such modifications by acting as regulators of key antioxidant gene expression. These findings suggest that antioxidant genes are, almost in part, under the control of sex hormones, and that pathophysiology of the difference in gender disease may depend on the redox biology.
Keywords: Estrogen replacement therapy; Glutathione; Menopause; Oxidative stress.
Figures




References
-
- Al-Azzawi F., Palacios S. Hormonal changes during menopause. Maturitas. 2009;63:135–137. - PubMed
-
- Mercuro G., Zoncu S., Saiu F., Mascia M., Melis G.B., Rosano G.M. Menopause induced by oophorectomy reveals a role of ovarian estrogen on the maintenance of pressure homeostasis. Maturitas. 2004;47:131–138. - PubMed
-
- Ozdemir S., Celik C., Gorkemli H., Kiyici A., Kaya B. Compared effects of surgical and natural menopause on climacteric symptoms, osteoporosis, and metabolic syndrome. International Journal of Gynaecology and Obstetrics. 2009;106:57–61. - PubMed
-
- Parker W.H., Jacoby V., Shoupe D., Rocca W. Effect of bilateral oophorectomy on women's long-term health. Womens Health (London, England ) 2009;5:565–576. - PubMed
-
- Judd H.L., Lucas W.E., Yen S.S. Effect of oophorectomy on circulating testosterone and androstenedione levels in patients with endometrial cancer. American Journal of Obstetrics and Gynecology. 1974;118:793–798. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

