Perturbation of Brachypodium distachyon CELLULOSE SYNTHASE A4 or 7 results in abnormal cell walls
- PMID: 24024469
- PMCID: PMC3847494
- DOI: 10.1186/1471-2229-13-131
Perturbation of Brachypodium distachyon CELLULOSE SYNTHASE A4 or 7 results in abnormal cell walls
Abstract
Background: Cellulose is an integral component of the plant cell wall and accounts for approximately forty percent of total plant biomass but understanding its mechanism of synthesis remains elusive. CELLULOSE SYNTHASE A (CESA) proteins function as catalytic subunits of a rosette-shaped complex that synthesizes cellulose at the plasma membrane. Arabidopsis thaliana and rice (Oryza sativa) secondary wall CESA loss-of-function mutants have weak stems and irregular or thin cell walls.
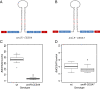
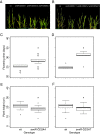
Results: Here, we identify candidates for secondary wall CESAs in Brachypodium distachyon as having similar amino acid sequence and expression to those characterized in A. thaliana, namely CESA4/7/8. To functionally characterize BdCESA4 and BdCESA7, we generated loss-of-function mutants using artificial microRNA constructs, specifically targeting each gene driven by a maize (Zea mays) ubiquitin promoter. Presence of the transgenes reduced BdCESA4 and BdCESA7 transcript abundance, as well as stem area, cell wall thickness of xylem and fibers, and the amount of crystalline cellulose in the cell wall.
Conclusion: These results suggest BdCESA4 and BdCESA7 play a key role in B. distachyon secondary cell wall biosynthesis.
Figures







References
-
- d'Yvoire MB, Bouchabke-Coussa O, Voorend W, Antelme S, Cézard L, Legée F, Lebris P, Legay S, Whitehead C, McQueen-Mason SJ. et al.Disrupting the cinnamyl alcohol dehydrogenase 1 gene (BdCAD1) leads to altered lignification and improved saccharification in Brachypodium distachyon. Plant J. 2012;73:496–508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

