Essential role of the molecular chaperone gp96 in regulating melanogenesis
- PMID: 24024552
- PMCID: PMC4002504
- DOI: 10.1111/pcmr.12165
Essential role of the molecular chaperone gp96 in regulating melanogenesis
Abstract
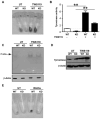
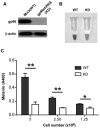
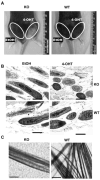
Through a process known as melanogenesis, melanocyte produces melanin in specialized organelles termed melanosomes, which regulates pigmentation of the skin, eyes, and hair. Gp96 is a constitutively expressed heat shock protein in the endoplasmic reticulum whose expression is further upregulated upon ultraviolet irradiation. However, the roles and mechanisms of this chaperone in pigmentation biology are unknown. In this study, we found that knockdown of gp96 by RNA interference significantly perturbed melanin synthesis and blocked late melanosome maturation. Gp96 knockdown did not impair the expression of tyrosinase, an essential enzyme in melanin synthesis, but compromised its catalytic activity and melanosome translocation. Further, mice with melanocyte-specific deletion of gp96 displayed decreased pigmentation. A mechanistic study revealed that the defect in melanogenesis can be rescued by activation of the canonical Wnt pathway, consistent with the critical roles of gp96 in chaperoning Wnt-coreceptor LRP6. Thus, this work uncovered the essential role of gp96 in regulating melanogenesis.
Keywords: Gp96; melanogenesis; melanosome; tyrosinase.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures



Similar articles
-
Melanosomal pH controls rate of melanogenesis, eumelanin/phaeomelanin ratio and melanosome maturation in melanocytes and melanoma cells.Exp Cell Res. 2001 Aug 1;268(1):26-35. doi: 10.1006/excr.2001.5251. Exp Cell Res. 2001. PMID: 11461115
-
Inhibition of melanin synthesis and melanosome transfer by chitosan biomaterials.J Biomed Mater Res B Appl Biomater. 2020 May;108(4):1239-1250. doi: 10.1002/jbm.b.34472. Epub 2019 Aug 22. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31436375
-
FK506 regulates pigmentation by maturing the melanosome and facilitating their transfer to keratinocytes.Pigment Cell Melanoma Res. 2016 Mar;29(2):199-209. doi: 10.1111/pcmr.12443. Epub 2016 Jan 27. Pigment Cell Melanoma Res. 2016. PMID: 26581186
-
Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation.Int J Cosmet Sci. 2018 Aug;40(4):328-347. doi: 10.1111/ics.12466. Epub 2018 Jul 19. Int J Cosmet Sci. 2018. PMID: 29752874 Review.
-
Recent advances in understanding the molecular basis of melanogenesis in melanocytes.F1000Res. 2020 Jun 15;9:F1000 Faculty Rev-608. doi: 10.12688/f1000research.24625.1. eCollection 2020. F1000Res. 2020. PMID: 32595944 Free PMC article. Review.
Cited by
-
Protein quality control in the endoplasmic reticulum.F1000Prime Rep. 2014 Jul 8;6:49. doi: 10.12703/P6-49. eCollection 2014. F1000Prime Rep. 2014. PMID: 25184039 Free PMC article. Review.
-
N-(4-methoxyphenyl) caffeamide-induced melanogenesis inhibition mechanisms.BMC Complement Altern Med. 2017 Jan 23;17(1):71. doi: 10.1186/s12906-016-1554-6. BMC Complement Altern Med. 2017. PMID: 28114924 Free PMC article.
-
Sageretia thea fruit extracts rich in methyl linoleate and methyl linolenate downregulate melanogenesis via the Akt/GSK3β signaling pathway.Nutr Res Pract. 2018 Feb;12(1):3-12. doi: 10.4162/nrp.2018.12.1.3. Epub 2018 Jan 18. Nutr Res Pract. 2018. PMID: 29399291 Free PMC article.
-
Differential Expression of Serum Exosomal Hsa-miR-487b-3p in Progressive Vitiligo Before and After Systemic Corticosteroid Treatment.Clin Cosmet Investig Dermatol. 2022 Jul 18;15:1377-1386. doi: 10.2147/CCID.S372112. eCollection 2022. Clin Cosmet Investig Dermatol. 2022. PMID: 35880009 Free PMC article.
-
Distinct patterns of proteostasis network gene expression are associated with different prognoses in melanoma patients.Sci Rep. 2024 Jan 2;14(1):198. doi: 10.1038/s41598-023-50640-0. Sci Rep. 2024. PMID: 38167612 Free PMC article.
References
-
- ABDEL-MALEK ZA, KADEKARO AL, SWOPE VB. Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 2010;23:171–86. - PubMed
-
- AGAR N, YOUNG AR. Melanogenesis: a photoprotective response to DNA damage? Mutat Res. 2005;571:121–32. - PubMed
-
- BASRUR V, YANG F, KUSHIMOTO T, HIGASHIMOTO Y, YASUMOTO K, VALENCIA J, MULLER J, VIEIRA WD, WATABE H, SHABANOWITZ J, et al. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J Proteome Res. 2003;2:69–79. - PubMed
-
- BELLEI B, FLORI E, IZZO E, MARESCA V, PICARDO M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal. 2008;20:1750–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

