Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia
- PMID: 24024753
- PMCID: PMC3960971
- DOI: 10.1513/AnnalsATS.201305-110OC
Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia
Abstract
Rationale: Several studies suggest that nasal nitric oxide (nNO) measurement could be a test for primary ciliary dyskinesia (PCD), but the procedure and interpretation have not been standardized.
Objectives: To use a standard protocol for measuring nNO to establish a disease-specific cutoff value at one site, and then validate at six other sites.
Methods: At the lead site, nNO was prospectively measured in individuals later confirmed to have PCD by ciliary ultrastructural defects (n = 143) or DNAH11 mutations (n = 6); and in 78 healthy and 146 disease control subjects, including individuals with asthma (n = 37), cystic fibrosis (n = 77), and chronic obstructive pulmonary disease (n = 32). A disease-specific cutoff value was determined, using generalized estimating equations (GEEs). Six other sites prospectively measured nNO in 155 consecutive individuals enrolled for evaluation for possible PCD.
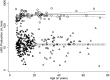
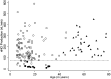
Measurements and main results: At the lead site, nNO values in PCD (mean ± standard deviation, 20.7 ± 24.1 nl/min; range, 1.5-207.3 nl/min) only rarely overlapped with the nNO values of healthy control subjects (304.6 ± 118.8; 125.5-867.0 nl/min), asthma (267.8 ± 103.2; 125.0-589.7 nl/min), or chronic obstructive pulmonary disease (223.7 ± 87.1; 109.7-449.1 nl/min); however, there was overlap with cystic fibrosis (134.0 ± 73.5; 15.6-386.1 nl/min). The disease-specific nNO cutoff value was defined at 77 nl/minute (sensitivity, 0.98; specificity, >0.999). At six other sites, this cutoff identified 70 of the 71 (98.6%) participants with confirmed PCD.
Conclusions: Using a standardized protocol in multicenter studies, nNO measurement accurately identifies individuals with PCD, and supports its usefulness as a test to support the clinical diagnosis of PCD.
Figures



Comment in
-
Nasal nitric oxide is an important test in the diagnostic pathway for primary ciliary dyskinesia.Ann Am Thorac Soc. 2013 Dec;10(6):645-7. doi: 10.1513/AnnalsATS.201309-328ED. Ann Am Thorac Soc. 2013. PMID: 24364768 Free PMC article. No abstract available.
-
A comparison of nasal nitric oxide measurement modes.Pediatr Pulmonol. 2017 Nov;52(11):1381-1382. doi: 10.1002/ppul.23780. Epub 2017 Aug 16. Pediatr Pulmonol. 2017. PMID: 28816018 Free PMC article. No abstract available.
References
-
- Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. - PubMed
-
- Brown DE, Pittman JE, Leigh MW, Fordham L, Davis SD. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol. 2008;43:514–516. - PubMed
-
- Barbato A, Frischer T, Kuehni CE, Snijders D, Azevedo I, Baktai G, Bartoloni L, Eber E, Escribano A, Haarman E, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34:1264–1276. - PubMed
-
- Papon JF, Coste A, Roudot-Thoraval F, Boucherat M, Roger G, Tamalet A, Vojtek AM, Amselem S, Escudier E. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2010;35:1057–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

