Inhibiting the HIV integration process: past, present, and the future
- PMID: 24025027
- PMCID: PMC3926363
- DOI: 10.1021/jm400674a
Inhibiting the HIV integration process: past, present, and the future
Erratum in
- J Med Chem. 2014 Jul 24;57(14):6273
Abstract
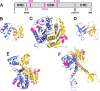
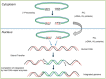
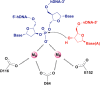
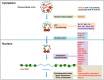
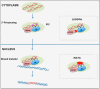
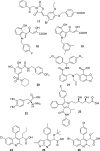
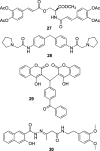
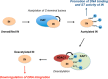
HIV integrase (IN) catalyzes the insertion into the genome of the infected human cell of viral DNA produced by the retrotranscription process. The discovery of raltegravir validated the existence of the IN, which is a new target in the field of anti-HIV drug research. The mechanism of catalysis of IN is depicted, and the characteristics of the inhibitors of the catalytic site of this viral enzyme are reported. The role played by the resistance is elucidated, as well as the possibility of bypassing this problem. New approaches to block the integration process are depicted as future perspectives, such as development of allosteric IN inhibitors, dual inhibitors targeting both IN and other enzymes, inhibitors of enzymes that activate IN, activators of IN activity, as well as a gene therapy approach.
Figures
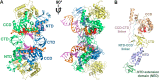
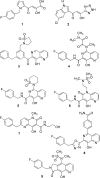
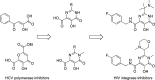
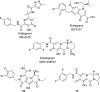
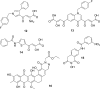
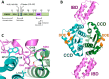
References
-
- Gottlieb M. S.; Schroff R.; Schanker H. M; Weisman J. D.; Fan P. T; Wolf R. A.; Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N. Engl. J. Med. 1981, 305, 1425–1431. - PubMed
-
- Masur H.; Michelis M. A.; Greene J. B.; Onorato I.; Stouwe R. A.; Holzman R. S.; Wormser G.; Brettman L.; Lange M.; Murray H. W.; Cunningham-Rundles S. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N. Engl. J. Med. 1981, 305, 1431–1438. - PubMed
-
- Siegal F. P.; Lopez C.; Hammer G. S.; Brown A. E.; Kornfeld S. J.; Gold J.; Hassett J.; Hirschman S. Z.; Cunningham-Rundles C.; Adelsberg B. R.; Parham D. M.; Siegal M.; Cunningham-Rundles S.; Armstrong D. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N. Engl. J. Med. 1981, 305, 1439–1444. - PubMed
-
- Cohen J. Therapies. Confronting the limits of success. Science 2002, 296, 2320–2324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

