Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration
- PMID: 24025096
- PMCID: PMC3847106
- DOI: 10.1186/1756-0500-6-366
Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration
Abstract
Background: Cell migration is a fundamental biological process and has an important role in the developing brain by regulating a highly specific pattern of connections between nerve cells. Cell migration is required for axonal guidance and neurite outgrowth and involves a series of highly co-ordinated and overlapping signalling pathways. The non-receptor tyrosine kinase, Focal Adhesion Kinase (FAK) has an essential role in development and is the most highly expressed kinase in the developing CNS. FAK activity is essential for neuronal cell adhesion and migration.
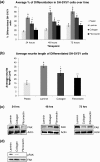
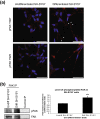
Results: The objective of this study was to optimise a protocol for the differentiation of the neuroblastoma cell line, SH-SY5Y. We determined the optimal extracellular matrix proteins and growth factor combinations required for the optimal differentiation of SH-SY5Y cells into neuronal-like cells and determined those conditions that induce the expression of FAK. It was confirmed that the cells were morphologically and biochemically differentiated when compared to undifferentiated cells. This is in direct contrast to commonly used differentiation methods that induce morphological differentiation but not biochemical differentiation.
Conclusions: We conclude that we have optimised a protocol for the differentiation of SH-SY5Y cells that results in a cell population that is both morphologically and biochemically distinct from undifferentiated SH-SY5Y cells and has a distinct adhesion and spreading pattern and display extensive neurite outgrowth. This protocol will provide a neuronal model system for studying FAK activity during cell adhesion and migration events.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

