Feasibility study on full closed-loop control ventilation (IntelliVent-ASV™) in ICU patients with acute respiratory failure: a prospective observational comparative study
- PMID: 24025234
- PMCID: PMC4056360
- DOI: 10.1186/cc12890
Feasibility study on full closed-loop control ventilation (IntelliVent-ASV™) in ICU patients with acute respiratory failure: a prospective observational comparative study
Abstract
Introduction: IntelliVent-ASV™ is a full closed-loop ventilation mode that automatically adjusts ventilation and oxygenation parameters in both passive and active patients. This feasibility study compared oxygenation and ventilation settings automatically selected by IntelliVent-ASV™ among three predefined lung conditions (normal lung, acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD)) in active and passive patients. The feasibility of IntelliVent-ASV™ use was assessed based on the number of safety events, the need to switch to conventional mode for any medical reason, and sensor failure.
Method: This prospective observational comparative study included 100 consecutive patients who were invasively ventilated for less than 24 hours at the time of inclusion with an expected duration of ventilation of more than 12 hours. Patients were ventilated using IntelliVent-ASV™ from inclusion to extubation. Settings, automatically selected by the ventilator, delivered ventilation, respiratory mechanics, and gas exchanges were recorded once a day.
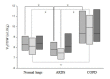
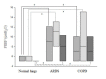
Results: Regarding feasibility, all patients were ventilated using IntelliVent-ASV™ (392 days in total). No safety issues occurred and there was never a need to switch to an alternative ventilation mode. The fully automated ventilation was used for 95% of the total ventilation time. IntelliVent-ASV™ selected different settings according to lung condition in passive and active patients. In passive patients, tidal volume (VT), predicted body weight (PBW) was significantly different between normal lung (n = 45), ARDS (n = 16) and COPD patients (n = 19) (8.1 (7.3 to 8.9) mL/kg; 7.5 (6.9 to 7.9) mL/kg; 9.9 (8.3 to 11.1) mL/kg, respectively; P 0.05). In passive ARDS patients, FiO2 and positive end-expiratory pressure (PEEP) were statistically higher than passive normal lung (35 (33 to 47)% versus 30 (30 to 31)% and 11 (8 to 13) cmH2O versus 5 (5 to 6) cmH2O, respectively; P< 0.05).
Conclusions: IntelliVent-ASV™ was safely used in unselected ventilated ICU patients with different lung conditions. Automatically selected oxygenation and ventilation settings were different according to the lung condition, especially in passive patients.
Trial registration: ClinicalTrials.gov: NCT01489085.
Figures

References
-
- East T, Heermann L, Bradshaw R, Lugo A, Sailors M, Ershler L, Wallace J, Morris A, McKinley B, Marquez A, Tonnesen A, Parmley L, Shoemaker W, Meade P, Thaut P, Hill T, Young M, Baughman J, Olterman M, Gooder V, Quinn B, Summer W, Valentine V, Carlson J, Bonnell B, deBoisblanc B, McClarity Z, Cachere J, Kovitz K, Gallagher E, Pinsky M, Efficacy of computerized decision support for mechanical ventilation. Results of a prospective multi-center randomized trial. Proc AMIA Symp. 1999. pp. 251–255. - PMC - PubMed
-
- Chatburn R, Mireles-Cabodevila E. Closed-loop control of mechanical ventilation: description and classification of targeting schemes. Respir Care. 2011;17:85–98. - PubMed
-
- Arnal JM, Wysocki M, Nafati C, Donati SY, Granier I, Corno G, Durand-Gasselin J. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med. 2008;17:75–81. - PubMed
-
- Sulzer CF, Chiolero R, Chassot PG, Mueller XM, Revelly JP. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology. 2001;17:1339–1345. - PubMed
-
- Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient-ventilator interactions during partial ventilatory support: A preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med. 2002;17:801–807. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

