A novel synthetic derivative of the natural product berbamine inhibits cell viability and induces apoptosis of human osteosarcoma cells, associated with activation of JNK/AP-1 signaling
- PMID: 24025361
- PMCID: PMC3925657
- DOI: 10.4161/cbt.26045
A novel synthetic derivative of the natural product berbamine inhibits cell viability and induces apoptosis of human osteosarcoma cells, associated with activation of JNK/AP-1 signaling
Abstract
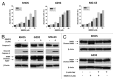
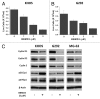
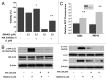
Osteosarcoma is the most common primary bone tumor in children and adolescents. There is a critical need to find more potent drugs for patients with metastatic or recurrent disease. Berbamine (BBM) is a natural compound derived from the Berberis amurensis plants. BBM and its derivatives have been shown to have antitumor effects in several cancers. Here, we report that a novel synthetic berbamine derivative, BBMD3, inhibits cell viability and induces apoptosis of G292, KHOS, and MG-63 human osteosarcoma cells. Induction of apoptosis in these tumor cells depends on activation of caspase-3 and cleavage of poly(ADP-ribose) polymerase (PARP). Since pan-caspase inhibitor (Z-VAD-FMK) and caspase-9 inhibitor (Z-LEHD-FMK) could block the cleavage of PARP, the apoptosis induced by BBMD3 is through intrinsic signaling pathway. BBMD3 increased phosphorylation of c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK), resulting in increase of phosphorylated c-Jun and total c-Fos, the major components of transcriptional factor AP-1. JNK inhibitor could partially suppress antitumor effect of BBMD3 on osteosarcoma cells. BBMD3 increased the production of reactive oxygen species (ROS) and ROS scavenger, N-acetylcysteine (NAC), could block the phosphorylation of JNK and c-Jun induced by BBMD3. BBMD3 increased the expression of the pro-apototic gene Bad, associated with apoptosis induction. Finally, BBMD3 also decreased the expression of cyclin D1 and D2, the positive cell cycle regulators, which is correlated with growth inhibition in osteosarcoma cells. Collectively, these findings indicate that BBMD3 is a potentially promising drug for the treatment of human osteosarcoma.
Keywords: AP-1; JNK; apoptosis; berbamine derivative; natural product; osteosarcoma.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
