Histone deacetylase 6-mediated deacetylation of α-tubulin coordinates cytoskeletal and signaling events during platelet activation
- PMID: 24025866
- PMCID: PMC3882361
- DOI: 10.1152/ajpcell.00053.2013
Histone deacetylase 6-mediated deacetylation of α-tubulin coordinates cytoskeletal and signaling events during platelet activation
Abstract
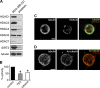
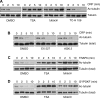
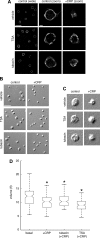
The tubulin cytoskeleton plays a key role in maintaining the characteristic quiescent discoid shape of resting platelets. Upon activation, platelets undergo a dramatic change in shape; however, little is known of how the microtubule system contributes to regulating platelet shape and function. Here we investigated the role of the covalent modification of α-tubulin by acetylation in the regulation of platelet physiology during activation. Superresolution microscopy analysis of the platelet tubulin cytoskeleton showed that the marginal band together with an interconnected web of finer tubulin structures collapsed upon platelet activation with the glycoprotein VI (GPVI)-agonist collagen-related peptide (CRP). Western blot analysis revealed that α-tubulin was acetylated in resting platelets and deacetylated during platelet activation. Tubacin, a specific inhibitor of the tubulin deacetylase HDAC6, prevented tubulin deacetylation upon platelet activation with CRP. Inhibition of HDAC6 upregulated tubulin acetylation and disrupted the organization of the platelet microtubule marginal band without significantly affecting platelet volume changes in response to CRP stimulation. HDAC6 inhibitors also inhibited platelet aggregation in response to CRP and blocked platelet signaling events upstream of platelet Rho GTPase activation. Together, these findings support a role for acetylation signaling in controlling the resting structure of the platelet tubulin marginal band as well as in the coordination of signaling systems that drive platelet cytoskeletal changes and aggregation.
Keywords: HDAC6; acetylation; platelets; tubulin.
Figures

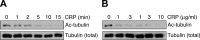



Comment in
-
Tubulin acetylation a valuable accessory of the platelet cytoskeleton. Focus on "Histone deacetylase 6-mediated deacetylation of α-tubulin coordinates cytoskeletal and signaling events during platelet activation".Am J Physiol Cell Physiol. 2013 Dec 15;305(12):C1211-3. doi: 10.1152/ajpcell.00309.2013. Epub 2013 Oct 9. Am J Physiol Cell Physiol. 2013. PMID: 24108865 No abstract available.
References
-
- Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell 100: 97–108, 2008 - PubMed
-
- Aslan JE, Itakura A, Gertz JM, McCarty OJ. Platelet shape change and spreading. Methods Mol Biol 788: 91–100, 2012 - PubMed
-
- Aslan JE, Itakura A, Haley KM, Tormoen GW, Loren CP, Baker SM, Pang J, Chernoff J, McCarty OJ. p21 activated kinase signaling coordinates glycoprotein receptor VI-mediated platelet aggregation, lamellipodia formation, and aggregate stability under shear. Arterioscler Thromb Vasc Biol 33: 1544–1551, 2013 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

