Early postweaning exercise improves central leptin sensitivity in offspring of rat dams fed high-fat diet during pregnancy and lactation
- PMID: 24026073
- PMCID: PMC3840316
- DOI: 10.1152/ajpregu.00566.2012
Early postweaning exercise improves central leptin sensitivity in offspring of rat dams fed high-fat diet during pregnancy and lactation
Abstract
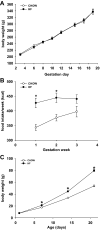
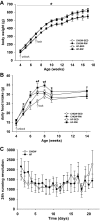
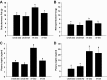
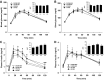
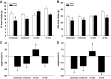
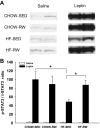
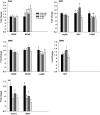
Maternal high-fat (HF) diet has long-term consequences on the metabolic phenotype of the offspring. Here, we determined the effects of postweaning exercise in offspring of rat dams fed HF diet during gestation and lactation. Pregnant Sprague-Dawley rats were maintained on chow or HF diet throughout gestation and lactation. All pups were weaned onto chow diet on postnatal day (PND) 21. At 4 wk of age, male pups were given free access to running wheels (RW) or remained sedentary (SED) for 3 wk, after which all rats remained sedentary, resulting in four groups: CHOW-SED, CHOW-RW, HF-SED, and HF-RW. Male HF offspring gained more body weight by PND7 compared with CHOW pups and maintained this weight difference through the entire experiment. Three weeks of postweaning exercise did not affect body weight gain in either CHOW or HF offspring, but reduced adiposity in HF offspring. Plasma leptin was decreased at the end of the 3-wk running period in HF-RW rats but was not different from HF-SED 9 wk after the exercise period ended. At 14 wk of age, intracerebroventricular injection of leptin suppressed food intake in CHOW-SED, CHOW-RW, and HF-RW, while it did not affect food intake in HF-SED group. At death, HF-RW rats also had higher leptin-induced phospho-STAT3 level in the arcuate nucleus than HF-SED rats. Both maternal HF diet and postweaning exercise had effects on hypothalamic neuropeptide and receptor mRNA expression in adult offspring. Our data suggest that postweaning exercise improves central leptin sensitivity and signaling in this model.
Keywords: development; exercise; leptin; maternal diet; obesity.
Figures
Similar articles
-
Large litter rearing improves leptin sensitivity and hypothalamic appetite markers in offspring of rat dams fed high-fat diet during pregnancy and lactation.Endocrinology. 2014 Sep;155(9):3421-33. doi: 10.1210/en.2014-1051. Epub 2014 Jun 13. Endocrinology. 2014. PMID: 24926823 Free PMC article.
-
Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity.Diabetes. 2012 Nov;61(11):2833-41. doi: 10.2337/db11-0957. Epub 2012 Jun 29. Diabetes. 2012. PMID: 22751689 Free PMC article.
-
Maternal and postweaning diet interaction alters hypothalamic gene expression and modulates response to a high-fat diet in male offspring.Am J Physiol Regul Integr Comp Physiol. 2009 Oct;297(4):R1049-57. doi: 10.1152/ajpregu.90585.2008. Epub 2009 Aug 5. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19657097
-
Animal models of maternal high fat diet exposure and effects on metabolism in offspring: a meta-regression analysis.Obes Rev. 2017 Jun;18(6):673-686. doi: 10.1111/obr.12524. Epub 2017 Mar 30. Obes Rev. 2017. PMID: 28371083 Free PMC article. Review.
-
Poor maternal nutrition during gestation in sheep alters key hormonal systems involved in energy homeostasis and appetite in the offspring.Domest Anim Endocrinol. 2025 Apr;91:106907. doi: 10.1016/j.domaniend.2024.106907. Epub 2024 Dec 10. Domest Anim Endocrinol. 2025. PMID: 39681045 Review.
Cited by
-
Leptin Signaling Could Mediate Hippocampal Decumulation of Beta-Amyloid and Tau Induced by High-Intensity Interval Training in Rats with Type 2 Diabetes.Cell Mol Neurobiol. 2023 Oct;43(7):3465-3478. doi: 10.1007/s10571-023-01357-1. Epub 2023 Jun 28. Cell Mol Neurobiol. 2023. PMID: 37378849 Free PMC article.
-
Neurodevelopmental Pathways to Obesity and Type 2 Diabetes: Insights From Prenatal Exposure to Maternal Obesity and Gestational Diabetes Mellitus: A Report on Research Supported by Pathway to Stop Diabetes.Diabetes. 2024 Dec 1;73(12):1937-1941. doi: 10.2337/dbi24-0012. Diabetes. 2024. PMID: 39432818 Review.
-
Rat offspring's microbiota composition is predominantly shaped by the postnatal maternal diet rather than prenatal diet.Physiol Behav. 2023 Jan 1;258:113987. doi: 10.1016/j.physbeh.2022.113987. Epub 2022 Oct 2. Physiol Behav. 2023. PMID: 36198343 Free PMC article.
-
Silkworm pupae powder ingestion increases fat metabolism in swim-trained rats.J Exerc Nutrition Biochem. 2014 Jun;18(2):141-9. doi: 10.5717/jenb.2014.18.2.141. Epub 2014 Feb 26. J Exerc Nutrition Biochem. 2014. PMID: 25566449 Free PMC article.
-
Corticosterone administration in drinking water decreases high-fat diet intake but not preference in male rats.Am J Physiol Regul Integr Comp Physiol. 2016 Apr 15;310(8):R733-43. doi: 10.1152/ajpregu.00371.2015. Epub 2016 Jan 27. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 26818055 Free PMC article.
References
-
- Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 35: 325–335, 2011 - PubMed
-
- Banks WA. Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Curr Pharm Des 9: 801–809, 2003 - PubMed
-
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53: 1253–1260, 2004 - PubMed
-
- Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides 20: 1341–1345, 1999 - PubMed
-
- Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab 285: E10–E15, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

