Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance
- PMID: 24026239
- PMCID: PMC3816630
- DOI: 10.1093/glycob/cwt077
Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance
Abstract
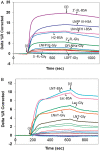
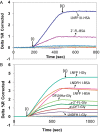
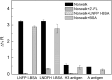
Human milk glycans inhibit binding between norovirus and its host glycan receptor; such competitive inhibition by human milk glycans is associated with a reduced risk of infection. The relationship between the presence of specific structural motifs in the human milk glycan and its ability to inhibit binding by specific norovirus strains requires facile, accurate and miniaturized-binding assays. Toward this end, a high-throughput biosensor platform was developed based on surface plasmon resonance imaging (SPRi) of glycan microarrays. The SPRi was validated, and its utility was tested, by measuring binding specificities between defined human milk glycan epitopes and the capsids of two common norovirus strains, VA387 and Norwalk. Human milk oligosaccharide (HMOS)-based neoglycoconjugates, including chemically derived neoglycoproteins and oligosaccharide-glycine derivatives, were used to represent polyvalent glycoconjugates and monovalent oligosaccharides, respectively, in human milk. SPRi binding results established that the glycan motifs that bind norovirus capsids depend upon strain; VA387 capsid interacts with two neoglycoproteins, whereas Norwalk capsid binds to a different set of HMOS motifs in the form of both polyvalent neoglycoproteins and monovalent oligosaccharides. SPRi competitive binding assays further demonstrated that specific norovirus-binding glycans are able to inhibit norovirus capsid binding to their host receptors. A polyvalent neoglycoconjugate with clustered carbohydrate moieties is required for the inhibition of VA387 capsid binding to host receptor glycans, whereas both monovalent oligosaccharides and polyvalent neoglycoconjugates are able to inhibit Norwalk capsid binding to its host receptor. Binding of HMOS and HMOS-based neoglycoconjugates to norovirus capsids depends upon the specific strain characteristics, implying that HMOS and their polyvalent derivatives are potential anti-adhesive agents for norovirus prophylaxis.
Keywords: anti-adhesives; host–pathogen interactions; human milk glycans; norovirus; surface plasmon resonance.
Figures

Similar articles
-
Human Norovirus Interactions with Histo-Blood Group Antigens and Human Milk Oligosaccharides.J Virol. 2016 Jun 10;90(13):5855-5859. doi: 10.1128/JVI.00317-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27122582 Free PMC article. Review.
-
Avidity of α-fucose on human milk oligosaccharides and blood group-unrelated oligo/polyfucoses is essential for potent norovirus-binding targets.J Biol Chem. 2018 Jul 27;293(30):11955-11965. doi: 10.1074/jbc.RA117.001369. Epub 2018 Jun 1. J Biol Chem. 2018. PMID: 29858242 Free PMC article.
-
Structural and binding studies of 2'- and 3-fucosyllactose and its complexes with norovirus capsid protein by molecular dynamics simulations.J Biomol Struct Dyn. 2023 Nov;41(19):10230-10243. doi: 10.1080/07391102.2022.2153923. Epub 2022 Dec 7. J Biomol Struct Dyn. 2023. PMID: 36476051
-
Fucoidan and Derived Oligo-Fucoses: Structural Features with Relevance in Competitive Inhibition of Gastrointestinal Norovirus Binding.Mar Drugs. 2021 Oct 21;19(11):591. doi: 10.3390/md19110591. Mar Drugs. 2021. PMID: 34822462 Free PMC article.
-
Structural insight of cell surface sugars in viral infection and human milk glycans as natural antiviral substance.Int J Biol Macromol. 2024 Oct;277(Pt 1):133867. doi: 10.1016/j.ijbiomac.2024.133867. Epub 2024 Jul 14. Int J Biol Macromol. 2024. PMID: 39009265 Review.
Cited by
-
Chemical-Shift Perturbations Reflect Bile Acid Binding to Norovirus Coat Protein: Recognition Comes in Different Flavors.Chembiochem. 2020 Apr 1;21(7):1007-1021. doi: 10.1002/cbic.201900572. Epub 2019 Dec 5. Chembiochem. 2020. PMID: 31644826 Free PMC article.
-
Synthesis of asymmetrical multiantennary human milk oligosaccharides.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):6954-6959. doi: 10.1073/pnas.1701785114. Epub 2017 Jun 19. Proc Natl Acad Sci U S A. 2017. PMID: 28630345 Free PMC article.
-
2'-Fucosyllactose Inhibits Human Norovirus Replication in Human Intestinal Enteroids.bioRxiv [Preprint]. 2024 May 30:2024.05.30.596597. doi: 10.1101/2024.05.30.596597. bioRxiv. 2024. Update in: J Virol. 2025 Feb 25;99(2):e0093824. doi: 10.1128/jvi.00938-24. PMID: 38853945 Free PMC article. Updated. Preprint.
-
Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health.J Funct Foods. 2020 Sep;72:104074. doi: 10.1016/j.jff.2020.104074. Epub 2020 Jul 3. J Funct Foods. 2020. PMID: 32834834 Free PMC article.
-
Human Norovirus Interactions with Histo-Blood Group Antigens and Human Milk Oligosaccharides.J Virol. 2016 Jun 10;90(13):5855-5859. doi: 10.1128/JVI.00317-16. Print 2016 Jul 1. J Virol. 2016. PMID: 27122582 Free PMC article. Review.
References
-
- Bejugam M, Flitsch SL. An efficient synthetic route to glycoamino acid building blocks for glycopeptide synthesis. Org Lett. 2004;6:001–4004. doi:10.1021/ol048342n. - DOI - PubMed
-
- Bu W, Mamedova A, Tan M, Xia M, Jiang X, Hegde RS. Structural basis for the receptor binding specificity of Norwalk virus. J Virol. 2008;82:5340–5347. doi:10.1128/JVI.00135-08. - DOI - PMC - PubMed
-
- Cao S, Lou ZY, Tan M, Chen YT, Liu YJ, Zhang ZS, Zhang XJC, Jiang X, Li XM, Rao ZH. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol. 2007;81:5949–5957. doi:10.1128/JVI.00219-07. - DOI - PMC - PubMed
-
- Cheng F, Shang J, Ratner DM. A versatile method for functionalizing surfaces with bioactive glycans. Bioconjug Chem. 2011;22:50–57. doi:10.1021/bc1003372. - DOI - PMC - PubMed
-
- Feng X, Jiang X. Library screen for inhibitors targeting norovirus binding to histo-blood group antigen receptors. Antimicrob Agents Chemother. 2007;51:324–331. doi:10.1128/AAC.00627-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

