Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance
- PMID: 24026239
- PMCID: PMC3816630
- DOI: 10.1093/glycob/cwt077
Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance
Abstract
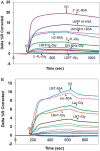
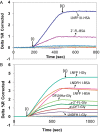
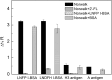
Human milk glycans inhibit binding between norovirus and its host glycan receptor; such competitive inhibition by human milk glycans is associated with a reduced risk of infection. The relationship between the presence of specific structural motifs in the human milk glycan and its ability to inhibit binding by specific norovirus strains requires facile, accurate and miniaturized-binding assays. Toward this end, a high-throughput biosensor platform was developed based on surface plasmon resonance imaging (SPRi) of glycan microarrays. The SPRi was validated, and its utility was tested, by measuring binding specificities between defined human milk glycan epitopes and the capsids of two common norovirus strains, VA387 and Norwalk. Human milk oligosaccharide (HMOS)-based neoglycoconjugates, including chemically derived neoglycoproteins and oligosaccharide-glycine derivatives, were used to represent polyvalent glycoconjugates and monovalent oligosaccharides, respectively, in human milk. SPRi binding results established that the glycan motifs that bind norovirus capsids depend upon strain; VA387 capsid interacts with two neoglycoproteins, whereas Norwalk capsid binds to a different set of HMOS motifs in the form of both polyvalent neoglycoproteins and monovalent oligosaccharides. SPRi competitive binding assays further demonstrated that specific norovirus-binding glycans are able to inhibit norovirus capsid binding to their host receptors. A polyvalent neoglycoconjugate with clustered carbohydrate moieties is required for the inhibition of VA387 capsid binding to host receptor glycans, whereas both monovalent oligosaccharides and polyvalent neoglycoconjugates are able to inhibit Norwalk capsid binding to its host receptor. Binding of HMOS and HMOS-based neoglycoconjugates to norovirus capsids depends upon the specific strain characteristics, implying that HMOS and their polyvalent derivatives are potential anti-adhesive agents for norovirus prophylaxis.
Keywords: anti-adhesives; host–pathogen interactions; human milk glycans; norovirus; surface plasmon resonance.
Figures

References
-
- Bejugam M, Flitsch SL. An efficient synthetic route to glycoamino acid building blocks for glycopeptide synthesis. Org Lett. 2004;6:001–4004. doi:10.1021/ol048342n. - DOI - PubMed
-
- Bu W, Mamedova A, Tan M, Xia M, Jiang X, Hegde RS. Structural basis for the receptor binding specificity of Norwalk virus. J Virol. 2008;82:5340–5347. doi:10.1128/JVI.00135-08. - DOI - PMC - PubMed
-
- Cao S, Lou ZY, Tan M, Chen YT, Liu YJ, Zhang ZS, Zhang XJC, Jiang X, Li XM, Rao ZH. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol. 2007;81:5949–5957. doi:10.1128/JVI.00219-07. - DOI - PMC - PubMed
-
- Cheng F, Shang J, Ratner DM. A versatile method for functionalizing surfaces with bioactive glycans. Bioconjug Chem. 2011;22:50–57. doi:10.1021/bc1003372. - DOI - PMC - PubMed
-
- Feng X, Jiang X. Library screen for inhibitors targeting norovirus binding to histo-blood group antigen receptors. Antimicrob Agents Chemother. 2007;51:324–331. doi:10.1128/AAC.00627-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

