Rapid, efficient and precise allele replacement in the fission yeast Schizosaccharomyces pombe
- PMID: 24026504
- PMCID: PMC3954454
- DOI: 10.1007/s00294-013-0406-x
Rapid, efficient and precise allele replacement in the fission yeast Schizosaccharomyces pombe
Abstract
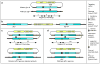
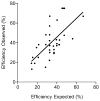
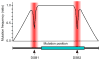
Gene targeting provides a powerful tool to modify endogenous loci to contain specific mutations, insertions and deletions. Precise allele replacement, with no other chromosomal changes (e.g., insertion of selectable markers or heterologous promoters), maintains physiologically relevant context. Established methods for precise allele replacement in fission yeast employ two successive rounds of transformation and homologous recombination and require genotyping at each step. The relative efficiency of homologous recombination is low and a high rate of false positives during the second round of gene targeting further complicates matters. We report that pop-in, pop-out allele replacement circumvents these problems. We present data for 39 different allele replacements, involving simple and complex modifications at seven different target loci, that illustrate the power and utility of the approach. We also developed and validated a rapid, efficient process for precise allele replacement that requires only one round each of transformation and genotyping. We show that this process can be applied in population scale to an individual target locus, without genotyping, to identify clones with an altered phenotype (targeted forward genetics). It is therefore suitable for saturating, in situ, locus-specific mutation screens (e.g., of essential or non-essential genes and regulatory DNA elements) within normal chromosomal context.
Figures


Similar articles
-
Targeted Forward Genetics: Population-Scale Analyses of Allele Replacements Spanning Thousands of Base Pairs in Fission Yeast.G3 (Bethesda). 2019 Dec 3;9(12):4097-4106. doi: 10.1534/g3.119.400805. G3 (Bethesda). 2019. PMID: 31597677 Free PMC article.
-
Targeted Forward Genetics: Saturating Mutational Analyses of Specific Target Loci Within the Genome.Methods Mol Biol. 2025;2862:223-239. doi: 10.1007/978-1-0716-4168-2_16. Methods Mol Biol. 2025. PMID: 39527204 Free PMC article.
-
Sequential and counter-selectable cassettes for fission yeast.BMC Biotechnol. 2016 Nov 8;16(1):76. doi: 10.1186/s12896-016-0307-4. BMC Biotechnol. 2016. PMID: 27825338 Free PMC article.
-
Homologous recombination in fission yeast: absence of crossover interference and synaptonemal complex.Experientia. 1994 Mar 15;50(3):295-306. doi: 10.1007/BF01924013. Experientia. 1994. PMID: 8143803 Review.
-
The meiotic recombination hotspots of Schizosaccharomyces pombe.Genome Dyn. 2009;5:1-13. doi: 10.1159/000166614. Genome Dyn. 2009. PMID: 18948703 Review.
Cited by
-
Targeted Forward Genetics: Population-Scale Analyses of Allele Replacements Spanning Thousands of Base Pairs in Fission Yeast.G3 (Bethesda). 2019 Dec 3;9(12):4097-4106. doi: 10.1534/g3.119.400805. G3 (Bethesda). 2019. PMID: 31597677 Free PMC article.
-
A CRISPR/Cas9-based method and primer design tool for seamless genome editing in fission yeast.Wellcome Open Res. 2017 May 5;1:19. doi: 10.12688/wellcomeopenres.10038.3. eCollection 2016. Wellcome Open Res. 2017. PMID: 28612052 Free PMC article.
-
Targeted Forward Genetics: Saturating Mutational Analyses of Specific Target Loci Within the Genome.Methods Mol Biol. 2025;2862:223-239. doi: 10.1007/978-1-0716-4168-2_16. Methods Mol Biol. 2025. PMID: 39527204 Free PMC article.
-
Implementation of the CRISPR-Cas9 system in fission yeast.Nat Commun. 2014 Oct 29;5:5344. doi: 10.1038/ncomms6344. Nat Commun. 2014. PMID: 25352017 Free PMC article.
-
Structure of the replication regulator Sap1 reveals functionally important interfaces.Sci Rep. 2018 Jul 19;8(1):10930. doi: 10.1038/s41598-018-29198-9. Sci Rep. 2018. PMID: 30026545 Free PMC article.
References
-
- Bach ML. Cloning and expression of the OMP decarboxylase gene URA4 from Schizosaccharomyces pombe. Curr Genet. 1987;12:527–534. - PubMed
-
- Bähler J, Wu J-Q, Longtine MS, Shah NG, McKenzie A, III, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

