MHF1-2/CENP-S-X performs distinct roles in centromere metabolism and genetic recombination
- PMID: 24026537
- PMCID: PMC3787749
- DOI: 10.1098/rsob.130102
MHF1-2/CENP-S-X performs distinct roles in centromere metabolism and genetic recombination
Retraction in
-
MHF1-2/CENP-S-X performs distinct roles in centromere metabolism and genetic recombination.Open Biol. 2018 Feb;8(2):180010. doi: 10.1098/rsob.180010. Open Biol. 2018. PMID: 29445032 Free PMC article. No abstract available.
Abstract
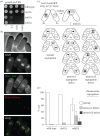
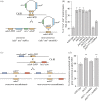
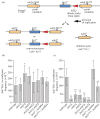
The histone-fold proteins Mhf1/CENP-S and Mhf2/CENP-X perform two important functions in vertebrate cells. First, they are components of the constitutive centromere-associated network, aiding kinetochore assembly and function. Second, they work with the FANCM DNA translocase to promote DNA repair. However, it has been unclear whether there is crosstalk between these roles. We show that Mhf1 and Mhf2 in fission yeast, as in vertebrates, serve a dual function, aiding DNA repair/recombination and localizing to centromeres to promote chromosome segregation. Importantly, these functions are distinct, with the former being dependent on their interaction with the FANCM orthologue Fml1 and the latter not. Together with Fml1, they play a second role in aiding chromosome segregation by processing sister chromatid junctions. However, a failure of this activity does not manifest dramatically increased levels of chromosome missegregation due to the Mus81-Eme1 endonuclease, which acts as a failsafe to resolve DNA junctions before the end of mitosis.
Keywords: DNA helicase; DNA replication; anaphase bridge; centromere; homologous recombination.
Figures





References
-
- Heyer WD, Ehmsen KT, Liu J. 2010. Regulation of homologous recombination in eukaryotes. Annu. Rev. Genet. 44, 113–139 (doi:10.1146/annurev-genet-051710-150955) - DOI - PMC - PubMed
-
- Meetei AR, et al. 2005. A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat. Genet. 37, 958–963 (doi:10.1038/ng1626) - DOI - PMC - PubMed
-
- Mosedale G, Niedzwiedz W, Alpi A, Perrina F, Pereira-Leal JB, Johnson M, Langevin F, Pace P, Patel KJ. 2005. The vertebrate Hef ortholog is a component of the Fanconi anemia tumor-suppressor pathway. Nat. Struct. Mol. Biol. 12, 763–771 (doi:10.1038/nsmb981) - DOI - PubMed
-
- Whitby MC. 2010. The FANCM family of DNA helicases/translocases. DNA Repair 9, 224–236 (doi:10.1016/j.dnarep.2009.12.012) - DOI - PubMed
-
- Kee Y, D'Andrea AD. 2010. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 24, 1680–1694 (doi:10.1101/gad.1955310) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
