Optical imaging of cortical networks via intracortical microstimulation
- PMID: 24027103
- PMCID: PMC3882772
- DOI: 10.1152/jn.00879.2012
Optical imaging of cortical networks via intracortical microstimulation
Abstract
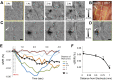
Understanding cortical organization is key to understanding brain function. Distinct neural networks underlie the functional organization of the cerebral cortex; however, little is known about how different nodes in the cortical network interact during perceptual processing and motor behavior. To study cortical network function we examined whether the optical imaging of intrinsic signals (OIS) reveals the functional patterns of activity evoked by electrical cortical microstimulation. We examined the effects of current amplitude, train duration, and depth of cortical stimulation on the hemodynamic response to electrical microstimulation (250-Hz train, 0.4-ms pulse duration) in anesthetized New World monkey somatosensory cortex. Electrical stimulation elicited a restricted cortical response that varied according to stimulation parameters and electrode depth. Higher currents of stimulation recruited more areas of cortex than smaller currents. The largest cortical responses were seen when stimulation was delivered around cortical layer 4. Distinct local patches of activation, highly suggestive of local projections, around the site of stimulation were observed at different depths of stimulation. Thus we find that specific electrical stimulation parameters can elicit activation of single cortical columns and their associated columnar networks, reminiscent of anatomically labeled networks. This novel functional tract tracing method will open new avenues for investigating relationships of local cortical organization.
Keywords: electrical microstimulation; intrinsic signal optical imaging.
Figures



References
-
- Bonhoeffer T, Grinvald A. Optical imaging based on intrinsic signals: the methodology. In: Brain Mapping: The Methods, edited by Toga AW, Mazziotta JC. San Diego, CA: Academic, 1996, p. 55–97
-
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig, Germany: Barth, 1909. (English translation by Laurence Garey as Brodmann's “Localisation in the Cerebral Cortex.” London: Smith-Cordon, 1994)
-
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eyefields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol 54: 714–734, 1985 - PubMed
-
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198, 2009 - PubMed
-
- Callaway EM. Local circuits in primary visual cortex of the macaque monkey. Annu Rev Neurosci 21: 47–74, 1998 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

