MiR-26b, upregulated in Alzheimer's disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons
- PMID: 24027266
- PMCID: PMC3810537
- DOI: 10.1523/JNEUROSCI.1327-13.2013
MiR-26b, upregulated in Alzheimer's disease, activates cell cycle entry, tau-phosphorylation, and apoptosis in postmitotic neurons
Abstract
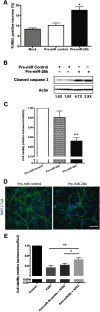
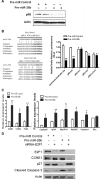
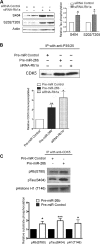
MicroRNA (miRNA) functions in the pathogenesis of major neurodegenerative diseases such as Alzheimer's disease (AD) are only beginning to emerge. We have observed significantly elevated levels of a specific miRNA, miR-26b, in the defined pathological areas of human postmortem brains, starting from early stages of AD (Braak III). Ectopic overexpression of miR-26b in rat primary postmitotic neurons led to the DNA replication and aberrant cell cycle entry (CCE) and, in parallel, increased tau-phosphorylation, which culminated in the apoptotic cell death of neurons. Similar tau hyperphosphorylation and CCE are typical features of neurons in pre-AD brains. Sequence-specific inhibition of miR-26b in culture is neuroprotective against oxidative stress. Retinoblastoma protein (Rb1), a major tumor suppressor, appears as the key direct miR-26b target, which mediates the observed neuronal phenotypes. The downstream signaling involves upregulation of Rb1/E2F cell cycle and pro-apoptotic transcriptional targets, including cyclin E1, and corresponding downregulation of cell cycle inhibitor p27/Kip1. It further leads to nuclear export and activation of Cdk5, a major kinase implicated in tau phosphorylation, regulation of cell cycle, and death in postmitotic neurons. Therefore, upregulation of miR-26b in neurons causes pleiotropic phenotypes that are also observed in AD. Elevated levels of miR-26b may thus contribute to the AD neuronal pathology.
Figures





References
-
- Arendt T, Holzer M, Gärtner U, Brückner MK. Aberrancies in signal transduction and cell cycle related events in Alzheimer's disease. J Neural Transm Suppl. 1998;54:147–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
