The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion
- PMID: 24027283
- PMCID: PMC3771024
- DOI: 10.1523/JNEUROSCI.1611-13.2013
The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion
Abstract
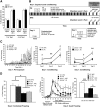
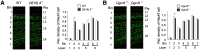
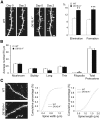
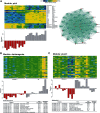
We used a mouse model of the schizophrenia-predisposing 22q11.2 microdeletion to evaluate how this genetic lesion affects cortical neural circuits at the synaptic, cellular, and molecular levels. Guided by cognitive deficits, we demonstrated that mutant mice display robust deficits in high-frequency synaptic transmission and short-term plasticity (synaptic depression and potentiation), as well as alterations in long-term plasticity and dendritic spine stability. Apart from previously reported reduction in dendritic complexity of layer 5 pyramidal neurons, altered synaptic plasticity occurs in the context of relatively circumscribed and often subtle cytoarchitectural changes in neuronal density and inhibitory neuron numbers. We confirmed the pronounced DiGeorge critical region 8 (Dgcr8)-dependent deficits in primary micro-RNA processing and identified additional changes in gene expression and RNA splicing that may underlie the effects of this mutation. Reduction in Dgcr8 levels appears to be a major driver of altered short-term synaptic plasticity in prefrontal cortex and working memory but not of long-term plasticity and cytoarchitecture. Our findings inform the cortical synaptic and neuronal mechanisms of working memory impairment in the context of psychiatric disorders. They also provide insight into the link between micro-RNA dysregulation and genetic liability to schizophrenia and cognitive dysfunction.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
