Effects of cathodal trans-spinal direct current stimulation on mouse spinal network and complex multijoint movements
- PMID: 24027294
- PMCID: PMC6705168
- DOI: 10.1523/JNEUROSCI.2793-13.2013
Effects of cathodal trans-spinal direct current stimulation on mouse spinal network and complex multijoint movements
Abstract
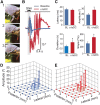
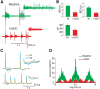
Cathodal trans-spinal direct current (c-tsDC) stimulation is a powerful technique to modulate spinal excitability. However, the manner in which c-tsDC stimulation modulates cortically evoked simple single-joint and complex multijoint movements is unknown. To address this issue, anesthetized mice were suspended with the hindlimb allowed to move freely in space. Simple and complex multijoint movements were elicited with short and prolonged trains of electrical stimulation, respectively, delivered to the area of primary motor cortex representing the hindlimb. In addition, spinal cord burst generators are known to be involved in a variety of motor activities, including locomotion, postural control, and voluntary movements. Therefore, to shed light into the mechanisms underlying movements modulated by c-tsDC stimulation, spinal circuit activity was induced using GABA and glycine receptor blockers, which produced three rates of spinal bursting activity: fast, intermediate, and slow. Characteristics of bursting activity were assessed during c-tsDC stimulation. During c-tsDC stimulation, significant increases were observed in (1) ankle dorsiflexion amplitude and speed; (2) ankle plantarflexion amplitude, speed, and duration; and (3) complex multijoint movement amplitude, speed, and duration. However, complex multijoint movement tracing showed that c-tsDC did not change the form of movements. In addition, spinal bursting activity was significantly modulated during c-tsDC stimulation: (1) fast bursting activity showed increased rate, amplitude, and duration; (2) intermediate bursting activity showed increased rate and duration, but decreased amplitude; and (3) slow bursting activity showed increased rate, but decreased duration and amplitude. These results suggest that c-tsDC stimulation amplifies cortically evoked movements through spinal mechanisms.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
