Mechanistic insights into the enhancement of adeno-associated virus transduction by proteasome inhibitors
- PMID: 24027330
- PMCID: PMC3838122
- DOI: 10.1128/JVI.01826-13
Mechanistic insights into the enhancement of adeno-associated virus transduction by proteasome inhibitors
Abstract
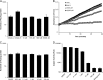
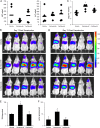
Proteasome inhibitors (e.g., bortezomib, MG132) are known to enhance adeno-associated virus (AAV) transduction; however, whether this results from pleotropic proteasome inhibition or off-target serine and/or cysteine protease inhibition remains unresolved. Here, we examined recombinant AAV (rAAV) effects of a new proteasome inhibitor, carfilzomib, which specifically inhibits chymotrypsin-like proteasome activity and no other proteases. We determined that proteasome inhibitors act on rAAV through proteasome inhibition and not serine or cysteine protease inhibition, likely through positive changes late in transduction.
Figures


References
-
- Binny C, McIntosh J, Della Peruta M, Kymalainen H, Tuddenham EG, Buckley SM, Waddington SN, McVey JH, Spence Y, Morton CL, Thrasher AJ, Gray JT, Castellino FJ, Tarantal AF, Davidoff AM, Nathwani AC. 2012. AAV-mediated gene transfer in the perinatal period results in expression of FVII at levels that protect against fatal spontaneous hemorrhage. Blood 119:957–966 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

