RNF115/BCA2 E3 ubiquitin ligase promotes breast cancer cell proliferation through targeting p21Waf1/Cip1 for ubiquitin-mediated degradation
- PMID: 24027428
- PMCID: PMC3769882
- DOI: 10.1593/neo.13678
RNF115/BCA2 E3 ubiquitin ligase promotes breast cancer cell proliferation through targeting p21Waf1/Cip1 for ubiquitin-mediated degradation
Abstract
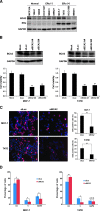
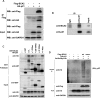
The E3 ubiquitin ligase RING finger protein 115 (RNF115), also known as breast cancer-associated gene 2 (BCA2), has previously been reported to be overexpressed in estrogen receptor α (ERα)-positive breast tumors and to promote breast cell proliferation; however, its mechanism is unknown. In this study, we demonstrated that silencing of BCA2 by small interfering RNAs (siRNAs) in two ERα-positive breast cancer cell lines, MCF-7 and T47D, decreases cell proliferation and increases the protein levels of the cyclin-dependent kinase inhibitor p21Waf/Cip1. The protein stability of p21 was negatively regulated by BCA2. BCA2 directly interacts with p21 and promotes p21 ubiquitination and proteasomal degradation. Knockdown of p21 partially rescues the cell growth arrest induced by the BCA2 siRNA. These results suggest that BCA2 promotes ERα-positive breast cancer cell proliferation at least partially through downregulating the expression of p21.
Figures



References
-
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. - PubMed
-
- Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, Elledge SJ. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–177. - PubMed
-
- Strohmaier H, Spruck CH, Kaiser P, Won KA, Sangfelt O, Reed SI. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature. 2001;413:316–322. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
